Article Text
Abstract
Objective Type 2 diabetes mellitus (T2DM) is one of the most prevalent chronic inflammatory diseases of the elderly. Its development is related to the alteration of the immune system with aging characterized by immunosenescence and inflamm-aging. In turn, T2DM also alters the immune response. As a consequence, older people with T2DM are more susceptible to influenza and to its complications as compared with healthy controls. Vaccination against influenza has shown poor efficacy in the older population and even less efficacy in patients with diabetes. We studied here the antibody response to vaccination in healthy and diabetic elderly participants.
Research design and methods In 2 groups of elderly participants (healthy N=119 and T2DM N=102), we measured the immunogenicity of influenza vaccine by hemagglutination inhibition assays. We assessed several blood and functional parameters as potential predictors of the vaccine efficacy.
Results We found no difference between antibody responses in diabetic elderly compared with healthy elderly. Among the biological and functional determinants, the cytomegalovirus (CMV) serostatus played a more prominent role in determining the magnitude of response. We concluded that in addition to age and diabetic status, immunological history such as CMV status should be taken into account. None of the other biological or functional parameters studied could be reliably linked to the vaccine antibody response in older adults who are not frail including those with well-controlled diabetes.
Conclusions Our data strongly suggest that influenza vaccine should be administered to elderly patients with T2DM; however, the immune determinants of the antibody response to influenza vaccination should be further investigated.
- Aging
- Vaccination
- Inflammation
- Biomarkers
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Key messages
No difference between antibody responses in diabetic elderly compared with healthy elderly.
Among the biological and functional determinants, the cytomegalovirus serostatus played a more prominent role in determining response intensity.
Influenza vaccine should be administered to elderly patients with type 2 diabetes mellitus.
Research questions
How the adaptive immune system respond to influenza vaccination in elderly patients with type 2 diabetes mellitus?
What is the role of the cytomegalovirus infection-induced inflammatory status for contributing to the better vaccine response?
Are there other biomarkers which influence the response to influenza vaccination?
Type 2 diabetes mellitus (T2DM) is one of the most prevalent chronic diseases of the elderly.1 Given that diabetes in older adults represents 50% of all cases of T2DM and that the prevalence of diabetes peaks at 15.5% in the age group of 75–79 years,2 the complications of diabetes will continue to increase with aging of the population.3 Risk for progressive disability is largely due to chronic diseases such as arthritis, diabetes, and peripheral vascular diseases,4 but whether this decline is preventable remains uncertain.5 In contrast, catastrophic disability has been attributed to discrete illness events including influenza and related complications including pneumonia, ischemic heart disease, congestive heart failure, and strokes,6 which represent four of the six leading causes of catastrophic disability7 and risk that could be more easily modified. To date, healthy diet, exercise, and vaccination remain the foundation for prevention in the older adult population.
Several physiological changes have been described during aging, and among them one of the most important is immunosenescence (the dysregulation of the immune system with aging) and the related effects of ‘inflamm-aging’.8 The age-related changes in the immune response affect mainly the adaptive compartment but innate immunity also changes.9 This dysregulation leads to the development of a low-grade inflammatory state which favors the appearance of age-related diseases including cardiovascular diseases, dementia, and T2DM.10 ,11 The causes of these immune changes with aging are not well defined. However, the contribution of thymic involution, chronic antigenic stimulation, especially by persistent cytomegalovirus (CMV) infection, and signalling/metabolic changes have been proposed.12 ,13 Thus, there is a common belief that chronic conditions such as T2DM further alter immune robustness in elderly individuals.14 Therefore, it is important to evaluate the efficiency of the immune response in healthy and T2DM elderly participants to solve these issues.
Influenza is a serious illness potentially leading to catastrophic disabilities in the elderly (defined as a loss of three or more basic activities of daily living in older adults).15 During the influenza seasonal peak, there is a significant excess of mortality from cardiovascular diseases, diabetes, pneumonia, and infections in general in the population aged 70 years and older.6 Epidemiological studies showed a rise in hospitalization and death rates due to influenza over the past two decades in spite of widespread influenza vaccination programmes16 which is not solely explained by the aging of the population. Furthermore, sequelae following hospitalizations for influenza illness17 are numerous which significantly and in an irreversible manner diminish the quality of life. The loss of independence in activities of daily living and the loss of as much as 50% of lower limb muscle strength are the major sequelae observed.18 Influenza is a preventable disease but older adults show hyporesponsiveness that may be associated with clinical conditions such as T2DM. Large studies have shown that age-related immune impairments include hyporesponsiveness to influenza vaccination by decreasing anti-influenza antibody titer, especially in elderly with T2DM.19 ,20 In contrast, influenza vaccination has consistently demonstrated a reduction in hospitalization rates for cardiovascular and pneumonia events in the elderly,21 and although this benefit has been questioned in older people with T2DM,22 recent studies however seem to suggest a real clinical and even immunological effectiveness of influenza vaccination in this population.23 ,24
Identified defects in immune functions related to diabetes are largely restricted to polymorphonuclear neutrophil function and a related increased risk for bacterial infections and candidiasis.25 Studies on the efficiency of the immune response to influenza vaccination in this population are limited and the biomarkers reflecting this efficiency are almost unknown.23 Thus, as the clinical effectiveness of influenza vaccination is well recognized, the aim of this study was to compare the antibody response to influenza vaccination in healthy elderly and older persons with optimally treated and well-controlled T2DM. The clinical and functional biomarkers determining the response to vaccination have also been investigated.
Research design and methods
Patients with T2DM and controls
A total of 221 participants aged ≥65 years from the communities of Greater Vancouver, British Columbia, and Sherbrooke, Quebec, were enrolled in this cross-sectional clinical study: 119 healthy participants chosen by the criteria of the SENIEUR protocol as already described and 102 participants with clinically well-controlled T2DM, treated only with oral hypoglycemic agents: 68 T2DM patients took metformin, 10 took sulfonylurea, and 24 took repaglinide. They were all community-dwelling; highly functional; and free of diabetic renal, eye, and neurological complications. Hypertension was medically treated in some patients but all of them received statin treatment. Furthermore, no participants suffered from heart failure, dementia, or cancer. No participant smoked. The clinical and functional characteristics of the groups studied are depicted in tables 1 and 2, respectively. All of the enrolled participants underwent influenza vaccination in the years 2010–2011 (healthy: 81 and T2DM: 61) and 2011–2012 (healthy: 38 and T2DM: 41). They received the standard dose of seasonal, trivalent, split-virus influenza vaccine. The vaccine in 2010–2011 and in 2011–2012 contained: A/California/7/2009 (H1N1), A/Victoria/210/2009 (H3N2), and B/Brisbane/60/2008. Patients were excluded in cases of overt cardiovascular disease and/or acute or chronic proven inflammatory diseases. Written informed consent for participation in the study was obtained from the patients and ethics approval was obtained from the local ethics committee for each site: the University of British Columbia of Clinical Research Ethics Board and the Research center on Aging (IRB: 2010–2020/Fulop).
Clinical parameters of the healthy elderly and elderly type 2 diabetes participants
Functional parameters of the healthy elderly and elderly type 2 diabetes participants
Study protocol
The volunteers were screened during a pre-enrolment visit where medical history and medication questionnaires were filled. Following screening, a blood sample was collected from the antecubital vein. The routine biological parameters such as WCC (white cell count) and red blood cell (RBC) count, glucose, liver and kidney functions, and glycated hemoglobin (HbA1c) were determined. Participants were screened and T2DM requiring insulin treatment, highly dysbalanced T2DM (glucose more than 12 mmol/L and HgbA1c >8.5% or 69 mmol/mol), or liver or kidney dysfunction (creatine clearance <30 mL/min) were excluded. All study participants had blood samples (40 mL) collected prior to intramuscularly administered influenza vaccine and at 28 days postvaccination (30 mL).
Biological parameters determination
All the routine biochemical laboratory tests were determined by the clinical laboratories for each of the study sites using the current best practices. The hemagglutination inhibition (HI) assay was performed using the recommendations of the WHO26 and as previously described.27 Briefly, we measured the immunogenicity of the vaccine in healthy and T2DM participants (≥65 years old) before (t0) and after vaccination (t28) by HI assay. Sera were stored frozen at −20°C. Paired preimmunization and postimmunization serum samples from the same individual were tested simultaneously, according to Frasca et al.23 Sera were pretreated with receptor-destroying enzyme (Denka Seiken Co Ltd) and serial twofold dilutions were performed and four HA units of the influenza vaccine strain were added with human RBCs (type 0).23 ,28 A fourfold rise in titer from prevaccination (t0) to postvaccination (t28) indicates seroconversion to the vaccine strain.29 The CMV serology was performed in the hospital virology laboratory of the University of Sherbrooke, and the McElhaney laboratory at the Vancouver Coastal Health Research Institute. The IgG levels against CMV were determined.
Functional parameters determination
The frailty index, which is based on a count of accumulated deficits divided by the number of potential deficits (80 items) was determined as described by Mitnitski et al30 (not frail, score <0.2; prefrail, score ≥0.2 and ≤0.3; frail, score >0.3). Grip strength was tested by a hand grip dynamometer using the average on three repeated measures for both hands following TNC-CDAAR procedure (http://cdaar.tufts.edu/protocols/Handgrip.pdf). Timed 6 m walk test was performed as described by Henwwod and Taafe,31 and expressed as time (s) per 6 m walked.
For the assessment of physical activity, a score ranging from 0 to 60 was assigned for physical activity using the Physical Activity Scale for the Elderly (PASE), and of frailty, a clinical frailty score (Canadian Study on Health and Aging (CSHA) Clinical Frailty Score, CFS) from 1 (completely healthy with no underlying chronic diseases) to 7 (completely dependent in all activities of daily living) were used.32 ,33
Statistical analyses
The distribution of demographic status including age and sex, the biological parameters including lipids, hematological, and inflammatory parameters were compared between the healthy and the T2DM cohorts, the CMV+ and CMV− cohorts, the responders and non-responders cohorts. The differences were determined using the χ2 test for categorical variables and the t test for continuous variables. We used the Pearson's or Spearman's correlation tests to establish correlations between the clinical status and the antibody response to vaccination measured by the change in hemagglutinin inhibition assay (HIA) titers from prevaccination to postvaccination. The Cox proportional hazard regression model was used to estimate the corresponding HR and 95% CI. Multivariate regression analysis was used so that, using the antibody response to vaccination as the dependent variable, we could determine which of the variables studied was independently associated with influenza vaccination. A value of p<0.05 was required for statistical significance. Data are reported as mean±SD.
Results
Inflammation and biological determinants in elderly patients with T2DM
Together, 221 older adults participated in the study, 119 healthy and 102 with T2DM. Their demographical and clinical parameters are presented in table 1. The two groups were matched for age. Significant differences were found in the lipid parameters as the mean total cholesterol and the low-density lipoprotein cholesterol (LDL-C) levels were lower in T2DM group compared with the healthy group, likely as a consequence of statin treatment. It is of note that all T2DM participants were under statin treatments while only 10% of the healthy elderly were under statin treatments. In contrast, the mean high-density lipoprotein cholesterol (HDL-C) level was also lower while the triglyceride (TG) level was higher in T2DM versus healthy older adults. We should note that although there were significant differences, all of these parameters were within the normal range. As expected, there were differences for glucose and HbA1c levels with a significant increase in T2DM. No differences between the inflammatory parameters measured by the commonly used C reactive protein and the less commonly used β2-microglobulin were found (table 1). The WCC count and concomitantly the absolute neutrophil number were significantly increased with T2DM, although within the normal range. Among the physical parameters, the body mass index (BMI) and waist circumference were significantly increased in T2DM, indicating overweight in this population (table 1). Thus, the healthy and T2DM groups were significantly different for the characteristic biological markers of T2DM, but not for others, such as lipids, probably due to the treatment of T2DM. No sign of chronic inflammation could be detected by these routine clinical measurements.
In addition to the biological parameters, functional tests are very important as a measure of the heterogeneity of health in aged individuals to assess the functional reserve. When we examined the functional parameters among healthy older and T2DM participants, we found no change in the physical performance measured by the 6 m walk test and hand grip, indicating good physical capacity in both groups. However, when we assessed the frailty in both groups as a marker of functional reserve, a significant difference was found using both frailty measurement tools, although the frailty index remained within the non-frail range. The PASE was significantly lower in the T2DM group indicating less physical activity but still within the normal range (table 2). These results suggest that older adults with T2DM have a tendency to be frailer and may not have the resiliency of healthy older adults in the face of an influenza infection.
The overall response to influenza vaccination is not affected by T2DM
Following vaccination against seasonal influenza, the healthy and the T2DM elderly were tested for their in vivo antibody response (figure 1). We found that the HIA ratio (day 28/day 0) did not differ between the two cohorts (figure 1A). This finding correlates with the few studies on this population.34 Next, we compared the number of participants arbitrarily identified as weak/non-responders 1<HIA<4 in the healthy elderly and in the T2DM group separately. We could not find any difference between the healthy and the T2DM groups (figure 1B). It is of note that the analysis of seroconversion rates (HIA ratio ≥4), considered to be a protective antibody response to vaccination, showed an equally low number of strong (true) responders in the healthy and T2DM groups with no significant difference between the groups (figure 1C).
The results of the influenza vaccination measured by hemagglutination inhibition assay (HIA) among healthy elderly and elderly patients with type 2 diabetes mellitus (T2DM). HIA was measured as described in Materials and methods. (A) Comparison of the HIA by ratio between days 28 and 0 (d28/0) among the total healthy elderly and T2DM elderly participants. (B) Distribution of weak responders (HIA≥1) versus non-responders at all (HIA=1) between non-T2DM (ND) and T2DM (D) elderly participants. (C) Distribution of the strong (true) responders, taking into account the accepted threshold for vaccination response between ND and D elderly participants. No significant difference was found at any comparison.
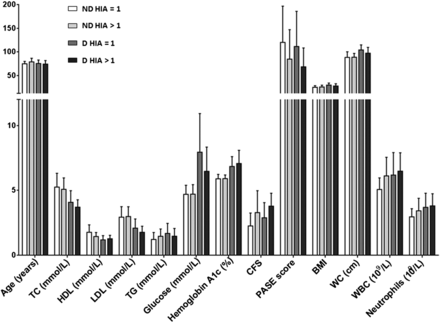
Next, we compared whether the parameters clinically and functionally measured between healthy and T2DM elderly participants differ among responders (HIA>1) and non-responders (HIA=1; figure 2). Thus, we did not find any difference between the responders and non-responders; however, the differences observed between healthy and diabetic participants for parameters such as glucose and HbA1c remained significantly different. No additional difference was observed with any other parameters.
Comparison of clinical and functional parameters among non-responders (=1) and responders (≥4) among healthy elderly and elderly patients with T2DM. No significant difference was found at any comparison. BMI, body mass index; CFS, Clinical Frailty Score; HIA, hemagglutination inhibition assay; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PASE, Physical Activity Scale for the Elderly; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglycerides; WC, waist circumference; WCC, white cell count.
We then investigated for vaccination efficiency-related parameters by looking for biomarkers correlated with the HIA day 28/day 0 ratio (table 3). The results indicated that, in the healthy group, the HIA ratio was negatively correlated with HDL-C and PASE and positively with WCC. In the T2DM group, the HIA ratio was negatively correlated with LDL-C, glucose, waist circumference, and with PASE. This suggests that the control of T2DM, such as glucose and the metabolic-related parameters, positively influences the response to influenza vaccination. Interestingly, we first noted, in the healthy elderly group, that the WCC was positively correlated with the HIA ratio. Moreover, in both groups, physical activity was negatively correlated with the HIA ratio, which may be explained by previous reports of physical activity having, in some circumstances, an immunosppressive effect.27 ,35 One other interesting finding in the T2DM group, was the negative correlation between the clinical frailty score (increasing frailty) and the antibody response to vaccination, even though the score was within the non-frail range. These results ultimately suggest that better diabetic control and less frailty may prevent hyporesponsiveness.
Correlation between the clinical and functional parameters with the response to the influenza vaccine measured by the ratio of HIA day 28/day 0
Response to vaccine is influenced by CMV seropositive status but not diabetes
As we could not find any clinically meaningful biomarkers linked to the antibody response to influenza vaccination in both groups, we investigated the potential role of immune exhaustion induced by chronic infections, such as CMV, in the amplitude of the antibody response to influenza vaccination (figure 3). First, we found no difference between the mean age of the CMV+ and CMV− participants (figure 3A). Moreover, the distribution of the number of CMV+ and CMV− participants within the two groups was also not different (figure 3B). Additionally, we wanted to verify whether the clinical and functional parameters changed regarding to the CMV serostatus. No difference was found for any of the studied parameters (see online supplementary figure S1). Thus, CMV does not determine per se any functional alteration in elderly individuals. When we separated the HIA response not between healthy and T2DM participants but between CMV+ and CMV− participants, we found significant difference in favor of a higher HIA ratio in CMV+ participants (p<0.0251; figure 3C). The fact that the CMV+ participants have a higher ratio of HIA was rather unexpected. Finally, we analyzed the HIA response to influenza vaccination on healthy and T2DM groups depending on their CMV serology. We found that, in any studied group, the CMV+ participants responded better that those CMV−, and this result was similar in the two study groups (figure 3D) suggesting that CMV seropositivity is more important than T2DM in the antibody response to influenza.
Age, cytomegalovirus (CMV) seropositivity in the healthy and type 2 diabetes mellitus (T2DM) and the response to influenza vaccination after the CMV serostatus in healthy and patients with T2DM. (A) Comparison of the age for CMV+ and CMV− elderly participants. No difference was found. (B) Distribution of the CMV− and CMV+ participants in the non-T2DM and T2DM elderly groups. No difference was found. (C) Hemagglutination inhibition assay (HIA) ratio day 28/day 0 (d28/d0) between the total CMV+ and CMV− elderly populations, which were significantly different (p<0.0251). (D) HIA ratio d28/d0 between CMV− and CMV+ participants in the non-T2DM and the T2DM elderly groups. CMV+ participants had significantly higher ratio (p<0.0482).
Repeating the same analyses as above, we divided the healthy and T2DM groups into CMV+ and CMV− individuals and then studied whether the clinical and functional parameters may distinguish participants in the healthy and the T2DM groups (figure 4). These results are in accordance with those found between the healthy and T2DM participants and as already showed (see online supplementary figure S1) CMV serostatus does not affect any of the biochemical parameters measured. However, as the CFS was significantly different between T2DM and healthy participants, independently of the CMV serostatus, we may assume that, in T2DM participants, diabetes is a more determinant factor for frailty than CMV serostatus, in contrast with healthy participants, for whom being CMV+ is more predictive of the frailty status. Interestingly, when a multiple regression analysis was performed none of these parameters emerged as more predictive for the frailty status (data not shown). However, these results suggest that for healthy participants, CMV seropositivity can be a driving force for frailty while in CMV+ participants, the T2DM is a further burden.
Comparison of clinical and functional parameters between the CMV+ and CMV− participants in the healthy elderly and elderly patients with T2DM. No difference was found at any comparison. Comparisons were made between the indicated groups having CMV− or CMV+ as well as being non-diabetic or diabetic. (*p<0.05; **p<0.01; ***p<0.001). BMI, body mass index; CFS, Clinical Frailty Score; CMV, cytomegalovirus; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PASE, Physical Activity Scale for the Elderly; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglycerides; WC, waist circumference; WCC, white cell count.
Finally, we wanted to investigate whether these biological and functional parameters found to be different among the CMV+ and CMV− healthy and T2DM participants may correlate with the various HIA levels (table 4). As expected, there was a correlation in CMV+ participants, independent of T2DM, between the CFS and the HIA ratio which could not be found in CMV− participants. Identical results were found for PASE. In CMV− participants, there was a negative correlation between the glucose level and the waist circumference and the immune response, suggesting that the control of diabetes in CMV− T2DM participants is crucial. In the CMV− healthy participants, the lipid parameters are negatively correlated with the response to vaccine. Altogether, these results show that the control of T2DM may be the most important parameter to improve the antibody response to influenza vaccination and that CMV+ participants show a more robust response.
Correlation between the HIAs and the different parameters in the CMV, diabetic, and healthy participant subgroups
Discussion
Aging comes with changes in the immune response, which translate into altered vaccination response.36 Aging is also associated with many chronic diseases including T2DM, and a shift toward low-grade inflammation (so-called ‘inflamm-aging’).37 The interaction between the effects of aging, chronic diseases, and inflamm-aging is postulated to inhibit the antibody response to influenza vaccination,38 but from a public health point of view, this is highly recommended.39 ,40 However, there have been very limited studies and have not shown a difference in healthy older adults compared with those with diabetes,23 but the vaccine coverage remains very low.41 Thus, our aim was to study the determinants of a robust response to influenza vaccination in older adults including important health indicators in those with T2DM.
We demonstrated in this study that the antibody response to influenza vaccination in older adults with well-controlled diabetes is not different from healthy elderly. However, there were very few seroconverters with a fourfold or greater rise in titer over the basal level (figure 1). These results are in contrast with the results of Frasca et al,23 showing that elderly patients with T2DM had in vivo and in vitro antibody responses even higher than those of healthy elderly age-matched controls. This apparent discrepancy may be explained by both the vaccination history and the control of the disease in these cohorts. Thus, we wanted to investigate whether overall health indicators, common biological markers, inflammatory parameters, and CMV serostatus might influence the magnitude of the vaccine response.42
A number of biological parameters characteristically altered in T2DM (lipids, glucose, HbA1c, WCC and neutrophil count, BMI, and waist circumference) showed significant differences in the healthy compared with the T2DM group despite having well-controlled T2DM and no clinically significant diabetes-related complications. What was surprising was the increased WCC and neutrophil count, albeit within the normal ranges, which may reflect low-level stimulation in the absence of differences in the serum inflammatory markers measured. These finding are in complete accordance with recent findings that increased neutrophil count may play an important role in age-associated chronic inflammatory diseases, such as Alzheimer disease and chronic heart failure.43 When functional parameters as a measure of overall health status were considered, we found lower levels of physical activity and increased frailty scores in the T2DM group, in line with what Fried and colleagues and others have reported.44 ,45
Diabetes is one of the chronic diseases where influenza vaccination is recommended and older adults with T2DM are less prone to influenza-induced clinical complications once vaccinated.46 Our study clearly shows that there is no difference in the HIA antibody response between healthy and T2DM participants; however, clinical and biological predictors of the antibody response differed between the two groups. In the healthy elderly, the HDL-C and the WCC counts, respectively, had a negative and positive association with the antibody response, parameters that have been already linked to age-induced dysregulation of the immune system.47 In the case of T2DM, only glucose remained as a strong determinant of the antibody response. It has been shown that hyperglycemia, overweight, and a lack of physical activity are correlated with a lower immune response.48 Hyperglycemia can impair neutrophil and monocyte/macrophage functions and suppress the T-cell response.46 Several recent studies indicate that physical activity impacts on immunity as it helps to clear the lungs from pathogens by increasing mucosal immunity, boosts antibody and cytokine secretion, and accelerates blood circulation;49 however, this can depend on the intensity and frequency of the activity and the basic immune status of the participants. Importantly, our results suggest that in well-controlled older patients with diabetes and in healthy older adults, level of frailty is an important predictor of the antibody response to influenza vaccination.
Based on studies showing that CMV infection plays a major role in immunosenescence,12 we explored whether CMV could play a role in the antibody response to influenza vaccination, either independently or in association with T2DM. We evaluated the entire study cohort according to their CMV serostatus and, very surprisingly, found that CMV+ participants responded significantly better than the CMV− participants. This seems counter intuitive knowing CMV-induced T cell senescence, however, a recent publication showed similar trend as in the present study.50 There is still a debate as to whether CMV+ versus CMV− individuals mount a lower response to influenza vaccination,51 ,52 although it has been shown that persistent CMV infection is associated with reduced responses to the influenza vaccine, and this seems to be mediated by higher percentages of late-differentiated CD4T cells in these individuals.53 However, we can perhaps hypothesize that CMV infection is accompanied by a higher level of the low-grade inflammation providing a stimulus to the immune response, the level of inflammation being higher in older adults with T2DM and with increased frailty as in the healthy older adults.54 Together, our data suggest that in well-controlled T2DM, CMV serostatus is an important determinant of the antibody response to influenza vaccination. Nevertheless, as the lower the lipid level is, better is the response in the CMV− population, it is possible that these molecules play a role in the response to influenza immunization. Thus, in the absence of CMV, the role of diabetic control including HDL levels47 ,55 should be included in future studies.
In conclusion, older adults with well-controlled T2DM mount antibody responses to influenza vaccination similar to those of healthy age-matched controls. Less frailty and improved control of diabetes may be the important determinants of vaccine responsiveness. CMV seropositivity is also associated with enhanced response to vaccination. It is worth to emphasize that these results may not be applicable to the much wider and more diverse population with T2DM, which is worthy to further study. Furthermore, since none of the biological or functional parameters studied were consistently linked to the antibody response to vaccination, further studies are also needed to investigate the immune determinants of the antibody response to influenza vaccination in older adults, especially with chronic diseases such as T2DM.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) (No. 106634 and No. 106701), the Université de Sherbrooke, and the Research Center on Aging and by grants NIH 5R21AI096446 and 1R21AG042826 (to Dr B Blomberg and Dr D Frasca). The technical assistance of Mrs Louise Rochon and Mrs Eliette Théberge is greatly acknowledged.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1 - Online supplement
Footnotes
Contributors This study was carried out in Vancouver and Sherbrooke, in Canada and co-authors are from Germany, Singapore and USA. JEM, TF, DF, AL, GP, GD, and EHF conceptualized the study, supervised the experiments, and wrote the paper; JEM, TF, and DT recruited and clinically evaluated the healthy elderly and the patients with diabetes. HG, XC, SB, and DF performed the experiments and participated to the analyses of the data. All authors read and approved the manuscript. TF is the guarantor.
Funding National Institute for Health Research; Canadian Institutes of Health Research.
Competing interests None declared.
Ethics approval The University of British Columbia of Clinical Research Ethics Board and the Research Center on Aging (IRB: 2010-20/Fulop).
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.