Abstract
Aims/hypothesis
The objective of the study was to analyse the mortality, survival and cause of death patterns in incident cases of diabetes in the 15–34-year age group that were reported to the nationwide prospective Diabetes Incidence Study in Sweden (DISS).
Materials and methods
During the study period 1983–1999, 6,771 incident cases were reported. Identification of deaths was made by linking the records to the nationwide Cause of Death Register.
Results
With an average follow-up of 8.5 years, resulting in 59,231 person-years, 159 deaths were identified. Diabetes was reported as the underlying cause of death in 51 patients (32%), and as a contributing cause of death in another 42 patients (26%). The standardised mortality ratio (SMR) was significantly elevated (RR=2.4; 95% CI: 2.0–2.8). The SMR was higher for patients classified by the reporting physician as having type 2 diabetes at diagnosis than for those classified as type 1 diabetic (2.9 and 1.8, respectively). Survival analysis showed significant differences in survival curves between males and females (p=0.0003) as well as between cases with different types of diabetes (p=0.005). This pattern was also reflected in the Cox regression model showing significantly increased hazard for males vs females (p=0.0002), and for type 2 vs type 1 (p=0.015) when controlling for age.
Conclusions/interpretation
This study shows a two-fold excess mortality in patients with type 1 diabetes and a three-fold excess mortality in patients with type 2 diabetes. Thus, despite advances in treatment, diabetes still carries an increased mortality in young adults, even in a country with a good economic and educational patient status and easy access to health care.
Similar content being viewed by others
Introduction
Excess mortality of patients with diabetes mellitus has been shown in several studies [1–3]. In children below the age of 15 years at diagnosis, studies based on the Swedish Childhood Diabetes Register have shown significantly increased short-term mortality [4, 5]. Similar results have been observed in other countries, e.g. in the United Kingdom for children diagnosed before the age of 17 years [6]. However, few studies of mortality in young adults are available.
Since 1983 a nationwide prospective registration of incident cases of diabetes mellitus in the 15–34-year age group has been ongoing in Sweden–the Diabetes Incidence Study in Sweden (DISS) [7]. The short-term mortality in DISS 1983–1987 and in DISS 1983–1992 showed a significant increase in all-cause mortality [8, 9]. Today we have access to mortality data through to 1999. This facilitates a study of both short- and long-term mortality. An extended analysis of the patterns of mortality is possible using survival analysis. Moreover, individual characteristics of the diabetic patients can be taken into account using Cox regression models, which have been used earlier in various studies of diabetes mellitus survival data [10, 11].
By linking the DISS records with the nationwide Cause of Death Registry, it is possible to identify deaths among the patients and obtain information on the date and cause of death. The aim of this study was to analyse the mortality, survival and cause of death patterns in incident cases of diabetes aged 15–34 years in relation to some basic characteristics at diagnosis.
Subjects and methods
The database
Since 1983 DISS has aimed to register all newly diagnosed diabetic patients in the 15–34-year age group. It is an ongoing prospective study conducted on a nationwide basis in collaboration with physicians in departments of endocrinology, internal medicine and paediatrics, and in primary health-care centres. In 1983 the mid-year populations in Sweden in the 5-year age groups 15–19, 20–24, 25–29 and 30–34 years were 607,200, 551,000, 566,400 and 602,000, respectively.
A standardised registration form is used with questions concerning patient’s name and address, ten-digit civic number including birth date and sex (a unique identity number given to all citizens at birth or at immigration), reporting primary health-care centre or hospital department, anthropometric measures (i.e. height and weight), basis for diagnosis (fasting and/or non-fasting blood glucose and/or 2-h OGTT), pregnancy, physician’s clinical classification of type of diabetes (type 1, type 2, secondary or unclassifiable), date of report and reporting physician. Since 1992 symptoms at diagnosis (polyuria and/or polydipsia, weight loss, fatigue, and duration of symptoms [in weeks]), ketonuria and acidosis, and treatment (diet, tablets or insulin) have been added to the form.
The level of ascertainment in DISS has been estimated in several studies. In the southern part of Sweden linkage of records between the Patient Administrative System for Inpatient Care and DISS 1983–1987 showed that the level of ascertainment for type 1 diabetes was good (86%) but that it was less good for type 2 diabetes (53%) [12]. A similar study in northern Sweden (county of Västerbotten) DISS 1986–1997 gave similar results, 91 and 49% for types 1 and 2 diabetes, respectively. The level of ascertainment of type 2 diabetes is poor because it has been difficult to keep in touch with about 1,000 primary health-care units in which there has been a high turnover of personnel during the study period. However, our studies of ascertainment levels did not indicate any systematic temporal or spatial bias.
The average yearly incidence for diabetes in Sweden in the 15–34-year age group between 1983 and 2001 was 22.3/100,000 for men and 14.3/100,000 for women; for patients classified as having type 1 diabetes, type 2 diabetes and unclassifiable diabetes the yearly incidences were 13.1, 3.1 and 1.6/100,000, respectively. There was no difference in the incidence by sex except for type 1 diabetes (16.6 vs 9.4/100,000 for men and women, respectively).
Between 1983 and 1999 6,807 patients were reported to DISS. Amongst these patients, 19 were excluded because of missing values of the type of diabetes and 17 were excluded because of missing or incomplete identification numbers. Of the remaining 6,771 patients, 4,968 (73%) were classified as having type 1 diabetes, 1,142 (17%) as having type 2 diabetes, 98 (1.4%) as having secondary diabetes, and 563 (8.3%) were considered unclassifiable by the reporting physician. Primary treatment was available for the time period 1992–1999; 54% of patients classified as having type 2 diabetes and 83% of patients with unclassifiable diabetes were treated with insulin.
Discrepancies between clinical classification and ‘objective’ criteria based on C-peptide have already been illustrated in the DISS 1983–1984 cohort [13]. In the 1987–1988 cohort we reported that 25% of type 2 diabetes patients had islet cell antibodies at diagnosis [14]. Similarly, in the 1991–1992 cohort [15] we reported that 25–30% of patients with type 2 diabetes had islet antibodies (islet cell antibodies, glutamic acid decarboxylase antibodies, or tyrosine phosphatase antibodies) and in the 1998 year cohort we reported that 25% of patients with clinical type 2 diabetes showed islet antibodies at diagnosis [16]. As we have also previously reported that follow-up of ‘type 2 diabetic’ patients with islet antibodies demonstrated beta cell failure and insulin dependency within 6 years after diagnosis [17] in these patients, we are well aware of the difficulties in defining type 1 or type 2 diabetes clinically. Thus, we have to accept that 25% of ‘type 2 diabetes’ patients should be considered as having type 1. Therefore, since 1998 islet antibodies have been measured at diagnosis in all DISS patients. However, during the current study period 1983–1999, islet antibodies were only measured during 1987–1988, 1991–1992 and 1998–1999 and can therefore not be considered for 11 of the 17 years. Hence, we are forced to use clinical criteria overall.
The study subjects gave informed consent for registration in DISS. The register has been approved by the Swedish Data Inspection Board and the Ethics Committee of the Karolinska Institute, Stockholm, Sweden.
Record linkage
The Swedish Cause of Death Registry has been computerised since 1961. Cause of death data by age, sex and year can be downloaded from the National Board of Health and Welfare homepage (http://www.sos.se). Underlying and contributory causes of death are assessed based on the cause on the death certificate and, if needed, on autopsy protocols and medical records. The validity of the underlying cause of death has been assessed in several studies and found to be high.
By linking DISS with the Cause of Death Registry until 31 December 1999 information on date and cause of death (underlying and contributing causes of death in terms of the International Classification of Diseases [ICD] classification codes ICD8, ICD9 and ICD10) was received for 159 cases. The median follow-up time was 8.5 years (range: 0–17), representing 59,231 person-years.
Statistical analysis
Mortality was measured with the indirect age-adjusted standardised mortality ratio (SMR) with the expected number of deaths calculated for persons of the same age and sex, i.e. general (Swedish) population. The calculation of the confidence intervals is based on the assumption that the number of deaths is Poisson-distributed over the time interval.
Survival analysis was performed by calculating survival functions with Kaplan–Meier estimates. Differences in survival curves were tested with the log-rank test. The impact on the risk of death of the characteristics of the patient and the type of diabetes at diagnosis was assessed by fitting a Cox proportional hazard model, where time to event is the time from date of diagnosis through to the end of follow-up on 31 December 1999 or death. The model’s assumptions (proportionality) were regularly checked.
All of the above mentioned calculations were made using the statistical software R (http://www.R-project.org).
A p value less than 0.05 was considered statistically significant.
Results
Mortality
During the 17-year study period 6771 patients were reported to DISS and at follow-up, on 31 December 1999, 159 had died, of whom 121 (76%) were men. The crude mortality rates were 168 (38/22,574) and 330 (121/36,657) per 100,000 person-years for women and men, respectively (Table 1).
Using the Swedish population as reference, 65.1 cases were expected to die, resulting in a significantly elevated SMR=2.4 (95% CI: 2.0–2.8) (Table 1). The SMR was significantly elevated for all types of diabetes with the highest risk for patients with secondary diabetes, SMR=19 (95% CI: 12–28). The SMR was slightly higher for patients with type 2 diabetes than for patients with type 1 diabetes (SMR=2.9 and 1.8, respectively). There was no difference in the values of SMR for type of diabetes by gender.
To analyse whether there was any trend in the SMR over time, the study period 1983–1999 was divided into cases diagnosed during two 6-year periods (1983–1988, 1989–1994) and one 5-year period (1995–1999) and followed through to 1988, 1994 and 1999, respectively (Table 2). The number of deaths in the three periods was 18, 9 and 6, respectively when cases with secondary diabetes were excluded. The SMR (3.3, 2.1 and 3.0, respectively) was significantly increased in all three periods but there was no time trend.
Cause of death
Besides diabetes, the most frequent underlying causes of death were circulatory diseases, suicide, violent deaths (excluding suicide) and cancer (Table 3). Four of 12 deaths from cancer were the result of a tumour in the pancreas (one patient with type 1 diabetes, one with unclassifiable diabetes and two with secondary diabetes). All 18 patients who committed suicide were men. Apart from suicide there were no apparent differences in the distribution of cause of death between men and women.
Diabetes was classified as the underlying cause of death in 51 (32%) of the 159 cases. Of patients diagnosed with type 1 diabetes, 36 (43%) had diabetes registered as the underlying cause of death. The corresponding figure for patients with type 2 diabetes was 5 (14%). Diabetes was reported as a contributing cause of death in another 42 cases (26%), the most common underlying cause of death in these patients being circulatory diseases (43%).
Survival
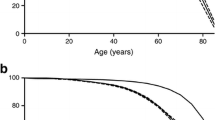
In the survival analysis, the 28 patients with secondary diabetes were excluded. The hypothesis of no differences in the survival curves between sexes was rejected (p=0.0003) (Fig. 1). A log-rank test extended for more than two groups under the hypothesis of no differences between different types of diabetes gave an observed chi-squared value on the test statistic of 10.8 with 2 df (p=0.005). The cumulative hazard functions stratified on type of diabetes showed an increased hazard for the patients with type 2 diabetes (Fig. 2).
The above-mentioned results are reflected in the Cox regression model described in Table 4. In the final model the explanatory variables gender, type of diabetes (as a factor) and age at diagnosis were included. For variables that are factors, the exponential function of the coefficient estimates is the ratio between the hazards of a group with the factor level of interest and a reference group. This hazard ratio can be interpreted as the relative death intensity. Table 4 shows that the death intensity for men vs women was 2.16. This can be seen as a reflection of the crude death rates of men and women in the background population, which were approximately 2 during the study period (Fig. 3). Similarly, the hazard ratio between the patients with type 2 diabetes and those with type 1 diabetes was 1.66. With age inserted as a covariate in the model, the intensity of dying was increased by 1.05 when the age was increased with 1 year. A likelihood ratio test for the model gives a chi-squared value of 33.9 with 4 df, which is significant (p<0.0001). Interaction terms inserted for possible interaction between age and type of diabetes were not significant in the model. Other factors that did not provide significant information on variation in hazard were BMI and public health region at diagnosis.
Discussion
This study is based on linkage of records between the nationwide prospective registers DISS and Cause of Death Registry. Earlier follow-ups to 1987 and 1992, with mean follow-up times of 2.5 and 5.0 years, respectively, resulted in SMRs of 2.1 and 2.3, respectively, for patients with type 1 diabetes at diagnosis and 4.8 and 4.1, respectively, for patients with type 2 diabetes at diagnosis [8, 9]. In this study the SMR for types 1 and 2 diabetes were 1.8 and 2.9 based on a mean follow-up time of 8.5 years. Thus this study confirms the earlier findings of a two-fold excess mortality for incident cases with type 1 diabetes and a threefold excess mortality for incident cases with type 2 diabetes in patients between 15 and 34 years at diagnosis.
Diabetes Incidence Study in Sweden (DISS)
DISS is based on reports from clinicians all over the country. The level of ascertainment is acceptable (>80%) [12]. Clinical classification of incident cases in this age group is difficult because 8.3% of patients were regarded by the reporting physician as impossible to classify [13]. This figure remained fairly stable during the study period. Three times during the study period, namely 1987–1988, 1992–1993 and 1998 onwards, reporting physicians have been offered free analysis of islet antibodies at the laboratory centre in Malmö. In general, one-quarter of patients with clinical type 2 diabetes showed islet cell antibodies and therefore probably had type 1 diabetes. It is therefore likely that the group of patients with type 1 diabetes is larger and that of patients with type 2 diabetes is smaller than reported; however, this is unlikely to affect the SMRs. However, as we only have access to islet cell antibodies at diagnosis for six out of the 17 year-cohorts we are forced to rely on the reporting physicians’ clinical classification.
In 1986–1987, 280 cases reported to DISS in 1983–1984 were followed-up by a questionnaire to the physician and by non-fasting C-peptide measurements [17]. Reclassification of type of diabetes (type 1, type 2 and unclassifiable) resulted in an overall kappa coefficient (a measure of the degree of non-random agreement between measurements of the same categorical variable) of 0.53 (95% CI: 0.43–0.63). For types 1 and 2 diabetes the kappa coefficients were 0.60 and 0.61, respectively, corresponding to moderate and good strength of agreement according to Landis and Koch’s definition [18].
The main epidemiological finding in DISS is the elevated incidence in men with type 1 diabetes compared with women (male:female ratio=1.8) which has been stable throughout the study period [19]. The peak incidence in the age group 15–34 years occurs in puberty after which the incidence drops, but much faster for females. For type 2 and unclassifiable cases the male:female ratio is 1.0 [20].
Quality of the cause of death certificates
The overall quality of the nationwide Swedish Cause of Death Registry, which has been computerised since 1961, has been extensively evaluated and found to be high [21–23]; however, diabetes is under-reported on death certificates and hence is underestimated by national mortality statistics [1, 23]. In the British Diabetic Association Cohort Study of 949 deaths among 23,752 patients with insulin-treated diabetes diagnosed before the age of 30 years and followed-up until 28 February 1997 with an average follow-up of 13.4 years, diabetes was mentioned as the cause of death on 67% of certificates and as an underlying cause in 36%, which is slightly higher than in our study (58 and 32%, respectively) [24].
This study is based on death from all causes, which implies that the under-reporting of diabetes on death certificates does not alter the findings expressed in the standard mortality ratios and the survival analysis. There are many aspects of the accuracy and quality of the information given on death certificates. All analyses of specific underlying or contributing causes of death are uncertain, especially in longitudinal studies during which changes in both coding practices and revisions of the ICD have taken place. Jansson et al. [25] concluded that statistics of published causes of death are profoundly influenced by these changes.
With the knowledge that diabetes in many cases is not mentioned on the death certificate and patients at risk are often lost to follow-up before the time of death, it is evident that a mortality study in diabetes must be prospective and population-based.
Risk for men vs women
The Cox regression showed that the death intensity for men vs women was 2.2. The ratio of the crude death rate for men vs women in the Swedish population aged 15–44 years has varied over the study period from 1.6 to 2.5 with a mean value of 2.1. Thus, the difference in hazard for men with diabetes compared with women with diabetes only reflects the increased general hazard in the Swedish population for men to die compared with women. This could also have been the case in a study in Denmark of a cohort of 906 patients diagnosed before 1943 at an age <31 years. These patients were followed-up and it was found that long-term survival among patients who had been aged 16–30 years at diagnosis was significantly better among women than men [26]. Similarly, in a study in Pittsburgh of 1,894 patients diagnosed between 1950 and 1981 at an age <17 years, SMR values of 11.5 and 5.4, respectively, were found in men and women [27]. In contrast, a study in southern Wisconsin of 1,210 cases with diabetes diagnosed before the age of 30 years arrived at SMRs of 6.8 and 8.9, respectively, for men and women [28]. However, all these studies are old. The British Diabetes Association Cohort Study [24] includes 23,752 patients diagnosed before the age of 30 years of whom 949 had died at follow-up in 1997 and who were followed for an average of 13.4 years. The overall SMRs for patients aged 20–49 years at death were 4.5–5.7 in women and 2.9–4.0 in men, thus considerably higher than our SMRs, in particular for women. This could be the result of the follow-up being longer by 5 years.
The higher hazard rate in men compared with women is not reflected in the SMRs (Table 1). This is because the expected number of deaths (the denominator of the SMR) is calculated based on the crude death rate in the general population which, during the study period, was on average twice as high for men as for women.
Risk for cases with type 1 vs type 2 diabetes
The fact that incident cases of type 2 diabetes can have carried the disease for several years before they are diagnosed might explain why the SMR for patients classified as having type 2 diabetes at diagnosis are slightly higher than the SMRs for cases with type 1 diabetes at diagnosis (SMR=2.9 and 1.8, respectively). In addition, patients with type 2 diabetes had a significantly higher BMI than the patients with type 1 diabetes. High BMI is a known risk factor for many circulatory diseases. This is manifested by a larger percentage of patients with circulatory diseases as underlying and contributory causes of death in type 2 compared with type 1 diabetes (51% [19/37] and 28% [23/83], respectively). To our knowledge, no study has managed to present separate SMRs for patients with types 1 and 2 diabetes diagnosed in the 15–34-year age group.
Secondary diabetes
The level of ascertainment was measured only for types 1 and 2 diabetes, implying that conclusions concerning mortality among secondary diabetes cases should be looked upon with care [11]. Considering the low number of participants classified with secondary diabetes it is likely that a substantial number of incident cases of secondary diabetes have not been reported to the DISS register.
Conclusion
This study has shown that diabetes in young adults is still a serious disease with increased mortality, particularly in those with type 2 diabetes.
Abbreviations
- DISS:
-
Diabetes Incidence Study in Sweden
- RR:
-
relative risk
- SMR:
-
standardised mortality ratio
References
Raymond NT, Langley JD, Goyder E et al (1995) Insulin-treated diabetes mellitus: causes of death determined from record linkage of population based registers in Leicestershire, UK. J Epidemiol Community Health 49:570–574
Koskinen SV, Reunanen AR, Martelin TP et al (1998) Mortality in a large population-based cohort of patients with drug-treated diabetes mellitus. Am J Public Health 88:765–770
Roper NA, Bilous RW, Kelly WF et al (2001) Excess mortality in a population with diabetes and the impact material deprivation: longitudinal, population-based study. Br Med J 322:1389–1393
Sartor G, Nyström L, Dahlquist G (1991) The Swedish childhood diabetes study: a seven-fold decrease in short-term mortality? Diabet Med 8:18–21
Sartor G, Dahlquist G (1995) Short-term mortality in childhood onset insulin-dependent diabetes mellitus: a high frequency of unexpected deaths in bed. Diabet Med 12:607–611
Warner DP, McKinney PA, Law GR et al (1998) Mortality and diabetes from a population-based register in Yorkshire 1978–93. Arch Dis Child 78:435–438
Östman J, Arnqvist H, Blohmé G et al (1986) Epidemiology of diabetes mellitus in Sweden: results of the first year of a prospective study in the population age group 15–34 years. Acta Med Scand 220:437–445
Nyström L, Östman J, Wall S et al (1992) Mortality of all incident cases of diabetes mellitus in Sweden diagnosed 1983–1987 at age 15–34 years. Diabet Med 9:422–427
Wibell L, Nyström L, Östman J et al (2001) Increased mortality in diabetes during the first 10 years of the disease. A population-based study (DISS) in Swedish adults 15–34 years old at diagnosis. J Intern Med 249:263–270
Andersen PK, Borch-Johnsen K, Deckert T et al (1985) A Cox regression model for the relative mortality and its application to diabetes mellitus survival data. Biometrics 41:921–932
Rossing P, Hougaard P, Borch-Johnsen K et al (1996) Predictors of mortality in insulin-dependent diabetes: 10 year observational follow up study. Br Med J 313:779–784
Littorin B, Sundkvist G, Scherstén B et al (1996) Patient administrative system as a tool to validate the ascertainment in the diabetes incidence study in Sweden (DISS). Diabet Res Clin Pract 33:129–133
Arnqvist HJ, Littorin B, Nyström L et al (1983) Difficulties in classifying diabetes at presentation in the young adult. Diabet Med 10:606–613
Landin-Olsson M, Karlsson FA, Lernmark Å, Sundkvist G, Diabetes Incidence Study in Sweden Group (1992) Islet cell and thyrogastric antibodies in 633 consecutive 15–34-yr old patients in the Diabetes Incidence Study in Sweden. Diabetes 41:1022–1027
Törn C, Landin-Olsson M, Östman J et al (2000) Glutamic acid decarboxylase antibodies (GADA) is the most important factor for prediction of insulin therapy within 3 years in young adult diabetic patients not classified as Type 1 diabetes on clinical grounds. Diab Metab Res Review 16:442–447
Borg H, Arnqvist HJ, Björk E et al (2003) Evaluation of the new ADA and WHO criteria for classification of diabetes mellitus in young adult people (15–34 yrs) in the diabetes incidence study in Sweden (DISS). Diabetologia 46:173–181
Littorin B, Sundkvist G, Hagopian W et al (1999) Islet cell and glutamic acid decarboxylase antibodies present at diagnosis of diabetes predict the need for insulin treatment. A cohort study in young adults whose disease was initially labeled as type 2 or unclassifiable diabetes. Diabetes Care 22:409–412
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Blohmé G, Nyström L, Arnqvist HJ et al (1992) Male predominance of Type 1 (insulin-dependent) diabetes mellitus in young adults: results from a 5-year prospective nationwide study of the 15–34 year age group in Sweden. Diabetologia 35:56–62
Nyström L, Dahlquist G, Östman J et al (1992). Risk of developing insulin-dependent diabetes mellitus (IDDM) before 35 years of age: indications of climatological determinants for age at onset. Int J Epidemiol 21:352–358
de Faire U, Friberg L, Lorich U, Lundman T (1976) A validation of cause-of-death certification in 1156 deaths. Acta Med Scand 200:223–228
Sundman L, Jakobsson S, Nyström L, Rosén M (1986) A validation of cause of death certification for ischemic heart disease in two Swedish municipalities. Scand J Prim Health Care 6:205–211
Andersson DKG, Svärdsudd K (1994) The value of death certification statistics in measuring mortality in persons with diabetes. Scand J Prim Health Care 12:114–120
Liang SP, Swerdlow AJ, Slater SD et al (1999) The British diabetic association cohort study I: all-cause mortality in patients with insulin-treated diabetes mellitus. Diabet Med 16:459–465
Jansson B, Johansson L A, Rosén M et al (1997) National adaptations of the ICD rules for classification—a problem in the evaluation of cause-of-death trends. J Clin Epidemiol 50:367–375
Borch-Johnsen K, Nissen H, Salling N, et al (1987) The natural history of insulin-dependent diabetes in Denmark: 2. Long-term survival—who and why. Diabet Med 4:211–216
Dorman JS, Laporte RE, Kuller LH et al (1984) The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study. Mortality study. Diabetes 33:271–276
Moss SE, Klein R, Klein BEK (1991) Cause-specific mortality in a population-based study of diabetes. Am J Public Health 81:1158–1162
Acknowledgements
This study was supported by grants from the Swedish Medical Research Council, the Swedish Diabetes Association, the Novo Nordisk Foundation, and the Juvenile Diabetes Foundation–Wallenberg Diabetes Research Programme (K98-99JD-128B).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00125-006-0242-3
Rights and permissions
About this article
Cite this article
Waernbaum, I., Blohmé, G., Östman, J. et al. Excess mortality in incident cases of diabetes mellitus aged 15 to 34 years at diagnosis: a population-based study (DISS) in Sweden. Diabetologia 49, 653–659 (2006). https://doi.org/10.1007/s00125-005-0135-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-0135-x