Abstract
Aims/hypothesis
Type 1 diabetes in children is characterised by autoimmune destruction of pancreatic beta cells and the presence of certain risk genotypes. In adults the same situation is often referred to as latent autoimmune diabetes in adults (LADA). We tested whether genetic markers associated with type 1 or type 2 diabetes could help to discriminate between autoimmune and non-autoimmune diabetes in young (15–34 years) and middle-aged (40–59 years) diabetic patients.
Methods
In 1,642 young and 1,619 middle-aged patients we determined: (1) HLA-DQB1 genotypes; (2) PTPN22 and INS variable-number tandem repeat (VNTR) polymorphisms; (3) two single nucleotide polymorphisms (rs7903146 and rs10885406) in the TCF7L2 gene; (4) glutamic acid decarboxylase (GAD) and IA-2-protein tyrosine phosphatase-like protein (IA-2) antibodies; and (5) fasting plasma C-peptide.
Results
Frequency of risk genotypes HLA-DQB1 (60% vs 25%, p = 9.4×10−34; 45% vs 18%, p = 1.4 × 10−16), PTPN22 CT/TT (34% vs 26%, p = 0.0023; 31% vs 23%, p = 0.034), INS VNTR class I/I (69% vs 53%, p = 1.3 × 10−8; 69% vs 51%, p = 8.5 × 10−5) and INS VNTR class IIIA/IIIA (75% vs 63%, p = 4.3 × 10−6; 73% vs 60%, p = 0.008) was increased in young and middle-aged GAD antibodies (GADA)-positive compared with GADA-negative patients. The type 2 diabetes-associated genotypes of TCF7L2 CT/TT of rs7903146 were significantly more common in young GADA-negative than in GADA-positive patients (53% vs 43%; p = 0.0004). No such difference was seen in middle-aged patients, in whom the frequency of the CT/TT genotypes of TCF7L2 was similarly increased in GADA-negative and GADA-positive groups (55% vs 56%).
Conclusions/interpretation
Common variants in the TCF7L2 gene help to differentiate young but not middle-aged GADA-positive and GADA-negative diabetic patients, suggesting that young GADA-negative patients have type 2 diabetes and that middle-aged GADA-positive patients are different from their young GADA-positive counterparts and share genetic features with type 2 diabetes.
Similar content being viewed by others
Introduction
Type 1 diabetes in children is mostly caused by an autoimmune process, characterised by T cell-mediated destruction of pancreatic beta cells. Common allelic variants at the HLA class II loci account for the major genetic risk in children and young adults [1–3]. Islet cell antibodies (ICA), glutamic acid decarboxylase antibodies (GADA), IA-2-protein tyrosine phosphatase-like protein antibodies (IA-2A) and insulin autoantibodies are established autoimmune markers of type 1 diabetes, but it is not clear whether they identify the same disease in young and adult diabetic patients [4–6]. It is generally thought that autoimmune diabetes in adults shows a slower progression towards insulin deficiency and no insulin requirement at diagnosis, which has led to the term latent autoimmune diabetes in adults (LADA) [7] or slowly progressing type 1 diabetes [8]. However, adult patients may also have rapid disease onset and ketoacidosis [9]. LADA is defined as GADA-positive diabetes in adults, but the definition of adult age has varied from 25 (UKPDS) to 40 years [10, 11]. Although type 1 diabetes is the predominant form of diabetes in young Europid diabetic patients, type 2 diabetes is increasing in young adults worldwide. TCF7L2 is by far the most important type 2 diabetes gene to date. The mechanisms by which it increases the risk of type 2 diabetes seem to include impaired beta cell function, possibly through an impaired incretin effect [12]. No association has been reported between variants in the TCF7L2 gene and type 1 diabetes [13].
Unfortunately, only few large studies have characterised antibody-positive diabetes in both young and middle-aged patients [11, 14, 15]. Also, to date, diagnosis of type 2 diabetes has been by exclusion, with patients not presenting with genetic and autoimmune markers of type 1 diabetes being considered to have type 2 diabetes [3, 11, 16, 17]. Apart from HLA, no other genetic markers have been used to distinguish type 1 and type 2 diabetes in young and middle-aged adults.
This study was designed to address the question of whether genetic markers (HLA-DQB1, INS variable-number tandem repeat (VNTR) alleles, PTPN22 and common variants in the TCF7L2 gene) can help distinguish between autoimmune and non-autoimmune diabetes in young (15–34 years) and middle-aged (40–59 years) patients.
Methods
Participants
In 1,642 of 1,824 diabetic patients (age at onset 15–34 years) from the Diabetes Incidence Study in Sweden (DISS) [3], islet antibodies (ICA, GADA, IA-2A) at diagnosis were measured. Fasting plasma C-peptide was measured at 3 to 6 months after diagnosis in 1,353 participants and HLA-DQB1, INS VNTR, PTPN22 and the TCF7L2 single nucleotide polymorphisms (SNPs), rs7903146 and rs10885406 were genotyped in 1,564 of these Swedish diabetic patients. In 1,619 of 1,636 diabetic patients (age at onset 40–59 years) from a local diabetes registry in southern Sweden (Scania Diabetes Registry), GADA were measured. Fasting plasma C-peptide was measured in 1,628 while HLA-DQB1 was genotyped in 1,365 and INS VNTR and PTPN22 in 1,312, and TCF7L2 in 1,614 patients. There was a 43% (701/1,632) overlap between the current data set of middle-aged type 2 diabetic patients from the Scania Diabetes Registry and a previous paper by Cervin et al. [18]. Informed consent was obtained from all patients.
A total of 216 non-diabetic participants from the county of Skaraborg, Sweden served as controls for HLA-DQB1 genotyping. In them, the HLA-DQB1 locus was amplified by PCR, followed by dot-blotting on to nitrocellulose filters and hybridisation using the radioactively end-labelled sequence-specific oligonucleotide probes, after which autoradiography was done [19].
In addition, non-diabetic control individuals (age at visit > 40 years) with no family history of diabetes or antihypertensive treatment were selected from the Malmö Preventive Project [20]. A total of 1,000 controls were available for INS VNTR (rs689 and rs3842755) and PTPN22 (rs2476601), and 11,923 controls for TCF7L2 (rs7903146 and rs10885406) genotyping.
Islet cell antibodies
ICA were determined in the young adult participants by a prolonged two-colour immunofluorescence assay [21]. The detection limit for ICA was 4 Juvenile Diabetes Foundation (JDF) units for the first pancreas used in samples tested until April 1999 and 5 JDF units for the second pancreas used in samples tested from April 1999 and onwards. In the last ICA Proficiency Test (13th) our ICA assay performed with 100% sensitivity and 100% specificity (ICA is not included in the Diabetes Autoantibody Standardization Program [DASP]).
GAD 65 antibodies
In the young participants GADA were measured by a radioligand binding assay, based on human 35S-labelled recombinant GAD 65 [22]. The results are presented as GADA index = 100 × (cpm of mean activity of all four measurements for sample − cpm of the negative control)/(cpm of the positive control − cpm of the negative control). A GADA index > 4.6 was considered as positive (97.5 percentile of 165 non-diabetic controls aged 7–34 years). In the first DASP (2000) the GADA assay showed a sensitivity of 80% and a specificity of 96%, in the second (2002) a sensitivity of 88% and a specificity of 87%, and in the third DASP (2003) a sensitivity of 82% and a specificity of 93%.
In the middle-aged participants GADA were measured by a radioligand binding assay using 35S-labelled recombinant human GAD 65 produced by in vitro transcription/translation [23]. The results are presented as relative units (RU): RU = 100 × (cpm of sample − mean cpm of three negative controls)/(cpm of a positive internal reference serum − mean cpm of three negative controls). The cut-off limit for positivity was 5 RU, which is comparable to 32 IU/ml according to the new WHO standard [24] and represents mean + 3 SD of 296 healthy control participants. In the first DASP (2000) the GADA assay showed a sensitivity of 76% and a specificity of 94%, in the second (2002) a sensitivity of 88% and a specificity of 96%, and in the third DASP (2003) a sensitivity of 88% and a specificity of 94% [25]. From the beginning of the year 2000 (in 41% of registry patients), the results were given as IU/ml [26].
IA-2-protein tyrosine phosphatase-like protein antibodies
IA-2A were measured in young adults by a similar assay based on human 35S-labelled recombinant IA-2 [27]. IA-2A index > 1.0 was considered as positive (97.5 percentile of 165 non-diabetic controls aged 7–34 years). In the first DASP (2000), the IA-2A assay showed a sensitivity of 58% and a specificity of 100%, in the second (2002) a sensitivity of 62% and a specificity of 100%, and in the third DASP (2003) a sensitivity of 64% and a specificity of 100%.
Plasma C-peptide
An RIA was used to determine fasting plasma C-peptide (Peninsula Laboratories, Belmont, CA, USA) in young (age 15–34 years) [3] and middle-aged (age 40–59 years) diabetic patients [26]. The reference range for healthy participants after 12 h fasting was 0.25 to 0.75 nmol/l.
HLA-DQB1 genotyping
Using a primer pair with biotinylated 3′ primers, the 158 bp second exon of HLA-DQB1 gene was amplified by PCR. The amplification product was bound to streptavadin-coated microtitration plates and denatured with NaOH. After washing, bound DNA was assessed using two different hybridisation mixtures with lanthanide (III) chelate-labelled DNA probes specific for the HLA-DQB1 alleles. One mixture contained europium (Eu)-labelled and samarium (Sm)-labelled internal reporter probes for DQB1*0602 and *0603 alleles (*0602–*0603) and DQB1*0603 and *0604 (*0603–*0604) alleles respectively; a terbium (Tb)-labelled consensus sequence-specific probe (Tb-DQB1 control) was used as control of PCR amplification. The other mixture contained Tb-, Sm- and Eu-labelled probes specific for DQB1*0201, DQB1*0301 and DQB1*0302 alleles, respectively. To measure probe hybridisation, microtitration plates were evaluated by time-resolved fluorescence (Delfia Research Fluorometer; Wallac OY, Turku, Finland). Different emission wavelengths and delay times were used to distinguish the signals of each lanthanide label [28]. From 1,564 genotyped DISS patients, genotyping success rate was 1,537 (98%) and from 1,365 diabetes registry patients 1,350 (99%). The risk HLA-DQB1 genotypes include *02/*0302, *0302/X, *0302/*604 [3] and in the following we refer to risk HLA-DQB1 without any further risk genotype specification.
VNTR polymorphisms in the insulin gene and in PTPN22 and TCF7L2 polymorphisms
Two SNPs associated with INS VNTR were genotyped, i.e. rs689 (−23HphI variant, a surrogate for the subdivision of VNTR into class I [A] and III [T] alleles) and rs3842755, which also allows subdivision of class III into IIIA (C) and IIIB (A) alleles [29–31]. The SNP rs2476601 (also denoted 1858C/T) was genotyped in the PTPN22 gene [32, 33]. Genotyping was performed by an allelic discrimination method (7900HT system; Applied Biosystems, Foster City, CA, USA). From young diabetic patients 1,564 DNA samples were available and the genotyping success rate was 98% for PTPN22, 98% for INS VNTR rs689 and 98% for INS VNTR rs3842755. In genotyped 1,312 middle-aged diabetic patients genotyping success rate was 99% for PTPN22, 98% for INS VNTR rs689 and 96% for INS VNTR rs3842755. Random samples (3.2%) for each SNP were re-genotyped, with reproducibility of 99.9%.
SNP rs7903146 in TCF7L2 gene, previously shown to be associated with type 2 diabetes, and a more recently described microsatellite marker, captured by SNP rs10885406, were genotyped [34, 35].
Genotyping was performed by an allelic discrimination method (7900HT system; Applied Biosystems). In young diabetic patients 1,564 DNA samples were available and the genotyping success rate was 98% for TCF7L2 rs7903146 and 98% for TCF7L rs10885406. In middle-aged diabetic patients 1,614 DNA samples were available and the genotyping success rate was 96% for TCF7L2 rs7903146 and 99% for TCF7L rs10885406. Random samples (3.2%) for each SNP were re-genotyped for quality control. The T allele of TCF7L2 rs7903146 was in strong linkage disequilibrium with the G allele of TCF7L2 rs10885406 (D’ = 0.99; r 2 = 1.0). (Electronic supplementary material [ESM] Table 1).
Statistical analysis
Comparison of genotype frequencies between GADA-positive and GADA-negative diabetic patients and non-diabetic controls was tested by the two-tailed Fisher’s exact test or χ 2 test with Bonferroni adjustment for multiple comparisons (the normal p value of 0.05 multiplied by the number of tests being performed). Odds ratios and 95% CIs were calculated as previously described [3]. Continuous data are presented as median and 1st and 3rd quartile. The significance of differences in continuous variables between groups was assessed by the non-parametric Mann–Whitney U test. All statistical tests were performed by SPSS version 13.0 (SPSS, Chicago, IL, USA) or JMP version 5 for MAC OS X (SAS Institute, Cary, NC, USA).
Results
Comparison between antibody-positive and antibody-negative young (15–34 years old) diabetic patients
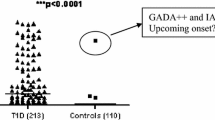
Out of 1,642 young diabetic patients, 1,013 (62%) had GADA, 829 (50%) had ICA and 716 (44%) had IA-2 antibodies. Of the young diabetic patients, 33% had all three antibodies (GADA, ICA, IA-2A), whereas 7.6% had either ICA or IA-2A or both, but not GADA and 31% were antibody-negative.
There was no sex-related difference between young antibody-positive and antibody-negative diabetic patients (ESM Table 2). Young antibody-positive patients had lower BMI (22 vs 29 kg/m2; p < 0.0001), were less likely to have a family history of diabetes (22% vs 43%; p = 2.9 × 10−13), were more frequently treated with insulin (93% vs 59%; p = 6.0 × 10−62) and had lower fasting plasma C-peptide concentrations (0.24 vs 0.70 nmol/l; p < 0.0001) than their antibody-negative counterparts. At diagnosis, 83% of the patients were on insulin therapy; this rose to 91% one month later when information on antibody status was available.
To allow comparison with GADA-positive middle-aged patients, we restricted the analysis of the young patients to the 1,013 GADA-positive participants (89% of all 1,138 antibody-positive patients) and excluded from the analysis those who were positive for only IA-2A or ICA or both. A full analysis including even these antibodies is given as ESM Tables 3 and 4.
There was no sex-related difference between young GADA-positive and GADA-negative diabetic patients (Table 1). Young GADA-positive patients had younger age at onset (25 vs 28 years; p < 0.0001), lower median BMI (22 vs 29 kg/m2; p < 0.0001), were less likely to have a family history of diabetes (23% vs 43%; p = 2.8 × 10−12), were more frequently treated with insulin (93% vs 59%; p = 1.1 × 10−57) and had lower median fasting plasma C-peptide concentrations (0.23 vs 0.70 nmol/l; p < 0.0001) than their GADA-negative counterparts (Table 1).
HLA-DQB1, PTPN22 and INS VNTR As expected, young GADA-positive diabetic patients had higher frequency of risk HLA-DQB1 (60% vs 25%; p = 9.4 ×10−34), PTPN22 CT/TT (34% vs 26%; p = 0.0023), INS VNTR (class I/I) AA (69% vs 53%; p = 1.3 × 10−8) and INS VNTR (class IIIA/IIIA) CC (75% vs 63%; p = 4.3 × 10−6) genotypes than young GADA-negative patients [36–39] (Table 2).
TCF7L2 The frequency of type 2 diabetes risk genotypes CT/TT of TCF7L2 (rs7903146) was significantly increased in young GADA-negative (53% vs 43%; p = 0.0004) compared with GADA-positive diabetic patients. Consequently, the frequencies of the wild-type CC genotypes were increased in young GADA-positive compared with GADA-negative patients (57% vs 47%) (Table 2).
To study whether the GADA level would influence the results, we re-analysed the data after dividing the GADA-positive patients into two subgroups with GADA levels below (low GADA) or above (high GADA) the median of GADA. Both groups showed the same increase in HLA DQB1 (59% and 60% vs 25%; p < 0.0001 for both) and the PTPN22 (34% and 33% vs 26%; p = 0.0044 and p = 0.013), INS VNTR (class I/I) AA (68% and 70% vs 53%; p < 0.0001 for both) and INS VNTR (class IIIA/IIIA) CC (73% and 76% vs 62%; p = 0.0008 and p < 0.0001) polymorphisms, as well as in CC genotypes of TCF7L2 (56% and 58% vs 47%; p = 0.0048 and p = 0.0011), as seen in the whole group compared with GADA-negative patients (ESM Table 5).
Comparison between middle-aged (40–59 years old) GADA-positive and GADA-negative diabetic patients
Out of 1,619 middle-aged diabetic patients, 205 (13%) were positive for GADA. There were more women among the GADA-positive than among GADA-negative middle-aged diabetic patients (49% vs 35%; p = 0.0001). GADA-positive patients also had lower median BMI (25 vs 29 kg/m2; p < 0.0001), were more frequently treated with insulin (60% vs 23%; p = 9.4 × 10−81) and had lower median fasting plasma C-peptide concentrations (0.27 vs 0.90 nmol/l; p < 0.0001) than their GADA-negative counterparts (Table 1). GADA-positive diabetic patients in this group had lower total cholesterol (median: 5.0 vs 5.3 mmol/l; p = 0.003), higher HDL-cholesterol (1.3 vs 1.0 mmol/l; p < 0.0001) and lower triacylglycerol (1.0 vs 1.8 mmol/l; p < 0.0001), but higher HbA1c (7.7% vs 7.1%; p < 0.0001) concentrations than GADA-negative patients.
HLA-DQB1, PTPN22 and INS VNTR Middle-aged GADA-positive patients also had a higher frequency of risk HLA-DQB1 (45% vs 18%; p = 1.4 × 10−16), PTPN22 CT/TT (31% vs 23%; p = 0.034), INS VNTR (class I/I) AA (69% vs 51%; p = 8.5 × 10−5) and INS VNTR (class IIIA/IIIA) CC (73% vs 60%; p = 0.008) genotypes than middle-aged GADA-negative patients (Table 2).
TCF7L2 There was no difference in type 2 diabetes-associated CT/TT genotypes of the TCF7L2 gene between middle-aged GADA-positive and GADA-negative patients (Table 2), both of whom showed a significantly higher frequency than controls (55% vs 43%; p = 1.5 × 10−9 and 24% vs 20%; p = 0.0007) (ESM Table 6).
We also subdivided the middle-aged GADA-positive group into two subgroups with GADA levels below (low GADA) or above (high GADA) the median. Both groups showed the same increase in HLA-DQB1 (55% and 36% vs 18%; p < 0.0001 and p < 0.0001) compared with GADA-negative patients. However, the frequency of INS VNTR (class I/I) AA (76% vs 51%; p < 0.0001) and INS VNTR (class IIIA/IIIA) CC (80% vs 60%; p = 0.003) was increased only in the high GADA compared with GADA-negative group. Notably, there was no difference in the frequency of the TCF7L2 CC genotypes between high and low GADA and GADA-negative groups, suggesting that the increase in the type 2 diabetes-associated CC genotypes was not restricted to those with low GADA levels (ESM Table 7).
Consequently, the frequency of the TCF7L2 risk genotypes was higher in middle-aged than in young GADA-positive diabetic patients (CT/TT: 55% vs 43%; p = 0.002; GG: 27% vs 19%; p = 0.01) suggesting that middle-aged GADA-positive patients have partially different genetic backgrounds (Table 3). The prevalence of common variants in the TCF7L2 gene did not differ between young and middle-aged GADA-negative diabetic patients (Table 3).
Discussion
We provide a comprehensive genetic and clinical characterisation of antibody-positive and -negative diabetes across the age groups 15 to 59 years, and one which is not inflated by subjective attempts to classify into type 1 and type 2 diabetes. The key finding of our study was that TCF7L2 CT/TT genotypes helped distinguish between young GADA-positive and GADA-negative patients (age 15–34 years) but not between middle-aged (40–59 years) GADA-positive and GADA-negative diabetic patients. These findings may have important implications for diagnosis of diabetic subgroups. The clinical diagnosis of young adults is not always easy and even young patients classified as having type 2 diabetes can have islet antibodies, a condition sometimes referred to as latent autoimmune diabetes in youth (LADY) [40]. Genetically, young and middle-aged antibody-positive patients were similar with respect to increased prevalence of risk genotypes HLA-DQB1, PTPN22 CT + TT, INS VNTR classI/I and INS VNTR classIIIA/IIIA. These findings are consistent with previous reports [3, 41, 42]. Although HLA genotyping together with measurements of islet antibodies has been used to identify individuals at risk of type 1 diabetes, until now no genetic markers have been available for type 2 diabetes. This has changed with the identification of the strong association between common variants in the TCF7L2 gene and type 2 diabetes, and may change further with the identification of novel genetic variants associated with type 2 diabetes in whole-genome association studies [34, 43–46]. Therefore, addition of TCF7L2 to HLA genotyping in young adults could clearly help to improve the prediction of diabetic subtype.
Our results in young GADA-positive patients on the frequency of SNPs in the TCF7L2 gene differ partially from recent findings in a large cohort of patients with type 1 diabetes [13]. While both studies showed no increase in the T allele of rs7903146 in apparent type 1 diabetic patients, we actually observed an increase of the C allele in GADA-positive young diabetic patients.
A possible explanation for this discrepancy could be age at onset of diabetes, which in the quoted study was below 17 years, but in our study ranged from 15 to 34 years. Under the age of 17 years type 2 diabetes is quite rare. In fact, when we subdivided the young adult patients into two age groups (15–25 and 25–34 years), we observed a stronger discriminatory effect of variants in the TCF7L2 gene in the older than in the younger group (ESM Table 8).
It is likely that our young GADA-negative patients do have true type 2 diabetes, as they shared the increased frequency of the type 2 diabetes-associated T allele in rs7903416 with middle-aged GADA-negative type 2 diabetic patients. In support of this, they also showed increased frequency of INS VNTR class III genotypes, which have previously been shown to be associated with type 2 diabetes [47, 48]. The increase of the C allele in the young GADA-positive patients could thus be a corollary of the increase of the T allele in the young GADA-negative patients. Alternatively, the C allele could in some way be associated with autoimmunity.
The TCF7L2 gene product is part of the Wnt signalling pathway, where after binding to beta-catenin, it activates transcription of a number of genes involved in cell proliferation [49, 50]. It is not known whether, like transcription factor 7, it influences Th1 responses through crosstalk with TGF-β signalling.
Our failure to find any difference in the frequency of the type 2 diabetes-associated T allele of rs7903416 in the TCF7L2 gene between middle-aged GADA-positive and GADA-negative patients, while finding a significantly higher frequency than in young GADA-positive patients, indicates that autoimmune diabetes in the middle-aged is different from autoimmune diabetes in young adults. These findings complement and add to our previous paper [18] showing that frequency of the TCF7L2 variants markedly differs between young and middle-aged autoimmune diabetes, further supporting the view that LADA represents an admixture of type 1 and type 2 diabetes. It also shows that if the age at onset for definition of LADA is lowered below 35 years, the LADA group will include an increasing number of patients with classical type 1 diabetes.
In conclusion, our findings suggest that common variants in the TCF7L2 gene can be used together with HLA-DQB1 genotyping to distinguish between young adults (15–34 years) with antibody-positive and antibody-negative diabetes. This is not possible in middle-aged (40–59 years) diabetic patients, suggesting that middle-aged antibody-positive patients are different from young antibody-positive patients and that autoimmune diabetes in middle-aged patients shares genetic features (common variants in the TCF7L2 gene) with type 2 diabetes.
Abbreviations
- DASP:
-
Diabetes Autoantibody Standardization Program
- DISS:
-
Diabetes Incidence Study in Sweden
- GADA:
-
glutamic acid decarboxylase antibodies
- IA-2A:
-
IA-2-protein tyrosine phosphatase-like protein antibodies
- ICA:
-
islet cell antibodies
- LADA:
-
latent autoimmune diabetes in adults
- RU:
-
relative units
- SNP:
-
single nucleotide polymorphism
- VNTR:
-
variable-number tandem repeat
References
Thorsby E, Ronningen KS (1993) Particular HLA-DQ molecules play a dominant role in determining susceptibility or resistance to type 1 (insulin-dependent) diabetes mellitus. Diabetologia 36:371–377
Sabbah E, Savola K, Kulmala P et al (1999) Disease-associated autoantibodies and HLA-DQB1 genotypes in children with newly diagnosed insulin-dependent diabetes mellitus (IDDM). The Childhood Diabetes in Finland Study Group. Clin Exp Immunol 116:78–83
Bakhtadze E, Borg H, Stenstrom G et al (2006) HLA-DQB1 genotypes, islet antibodies and beta cell function in the classification of recent-onset diabetes among young adults in the nationwide Diabetes Incidence Study in Sweden. Diabetologia 49:1785–1794
Groop L, Miettinen A, Groop PH, Meri S, Koskimies S, Bottazzo GF (1988) Organ-specific autoimmunity and HLA-DR antigens as markers for beta-cell destruction in patients with type II diabetes. Diabetes 37:99–103
Sanjeevi CB, Gambelunghe G, Falorni A, Shtauvere-Brameus A, Kanungo A (2002) Genetics of latent autoimmune diabetes in adults. Ann N Y Acad Sci 958:107–111
Vatay A, Rajczy K, Pozsonyi E et al (2002) Differences in the genetic background of latent autoimmune diabetes in adults (LADA) and type 1 diabetes mellitus. Immunol Lett 84:109–115
Tuomi T, Carlsson A, Li H et al (1999) Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes 48:150–157
Zimmet P, Turner R, McCarty D, Rowley M, Mackay I (1999) Crucial points at diagnosis. Type 2 diabetes or slow type 1 diabetes. Diabetes Care 22(Suppl 2):B59–B64
Maldonado M, Hampe CS, Gaur LK et al (2003) Ketosis-prone diabetes: dissection of a heterogeneous syndrome using an immunogenetic and beta-cell functional classification, prospective analysis, and clinical outcomes. J Clin Endocrinol Metab 88:5090–5098
Gottsater A, Landin-Olsson M, Lernmark A, Fernlund P, Sundkvist G, Hagopian WA (1995) Glutamate decarboxylase antibody levels predict rate of beta-cell decline in adult-onset diabetes. Diabetes Res Clin Pract 27:133–140
Turner R, Stratton I, Horton V et al (1997) UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet 350:1288–1293
Lyssenko V, Lupi R, Marchetti P et al (2007) Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 117:2155–2163
Field SF, Howson JM, Smyth DJ, Walker NM, Dunger DB, Todd JA (2007) Analysis of the type 2 diabetes gene, TCF7L2, in 13,795 type 1 diabetes cases and control subjects. Diabetologia 50:212–213
Wroblewski M, Gottsater A, Lindgarde F, Fernlund P, Sundkvist G (1998) Gender, autoantibodies, and obesity in newly diagnosed diabetic patients aged 40–75 years. Diabetes Care 21:250–255
Bruno G, De Salvia A, Arcari R et al (1999) Clinical, immunological, and genetic heterogeneity of diabetes in an Italian population-based cohort of lean newly diagnosed patients aged 30–54 years. Piedmont Study Group for Diabetes Epidemiology. Diabetes Care 22:50–55
Borg H, Arnqvist HJ, Bjork E et al (2003) Evaluation of the new ADA and WHO criteria for classification of diabetes mellitus in young adult people (15–34 yrs) in the Diabetes Incidence Study in Sweden (DISS). Diabetologia 46:173–181
Rotteveel J, Belksma EJ, Renders CM, Hirasing RA, Delemarre-Van de Waal HA (2007) Type 2 diabetes in children in the Netherlands: the need for diagnostic protocols. Eur J Endocrinol 157:175–180
Cervin C, Lyssenko V, Bakhtadze E et al (2008) Genetic similarities between latent autoimmune diabetes in adults, type 1 diabetes, and type 2 diabetes. Diabetes 57:1433–1437
Stenstrom G, Berger B, Borg H, Fernlund P, Dorman JS, Sundkvist G (2002) HLA-DQ genotypes in classic type 1 diabetes and in latent autoimmune diabetes of the adult. Am J Epidemiol 156:787–796
Nilsson PM, Nilsson JA, Berglund G (2004) Family burden of cardiovascular mortality: risk implications for offspring in a national register linkage study based upon the Malmo Preventive Project. J Intern Med 255:229–235
Olsson ML, Sundkvist G, Lernmark A (1987) Prolonged incubation in the two-colour immunofluorescence test increases the prevalence and titres of islet cell antibodies in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 30:327–332
Borg H, Fernlund P, Sundkvist G (1997) Measurement of antibodies against glutamic acid decarboxylase 65 (GADA): two new 125I assays compared with [35S]GAD 65-ligand binding assay. Clin Chem 43:779–785
Grubin CE, Daniels T, Toivola B et al (1994) A novel radioligand binding assay to determine diagnostic accuracy of isoform-specific glutamic acid decarboxylase antibodies in childhood IDDM. Diabetologia 37:344–350
Mire-Sluis AR, Das RG, Lernmark A (1999) The development of a World Health Organization international standard for islet cell antibodies: the aims and design of an international collaborative study. Diabetes Metab Res Rev 15:72–77
Verge CF, Stenger D, Bonifacio E et al (1998) Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes: Combinatorial Islet Autoantibody Workshop. Diabetes 47:1857–1866
Lindholm E, Hallengren B, Agardh CD (2004) Gender differences in GAD antibody-positive diabetes mellitus in relation to age at onset, C-peptide and other endocrine autoimmune diseases. Diabetes Metab Res Rev 20:158–164
Borg H, Fernlund P, Sundkvist G (1997) Protein tyrosine phosphatase-like protein IA2-antibodies plus glutamic acid decarboxylase 65 antibodies (GADA) indicates autoimmunity as frequently as islet cell antibodies assay in children with recently diagnosed diabetes mellitus. Clin Chem 43:2358–2363
Ilonen J, Reijonen H, Herva E et al (1996) Rapid HLA-DQB1 genotyping for four alleles in the assessment of risk for IDDM in the Finnish population. The Childhood Diabetes in Finland (DiMe) Study Group. Diabetes Care 19:795–800
Bennett ST, Lucassen AM, Gough SC et al (1995) Susceptibility to human type 1 diabetes at IDDM2 is determined by tandem repeat variation at the insulin gene minisatellite locus. Nat Genet 9:284–292
Barratt BJ, Payne F, Lowe CE et al (2004) Remapping the insulin gene/IDDM2 locus in type 1 diabetes. Diabetes 53:1884–1889
Desai M, Zeggini E, Horton VA et al (2006) The variable number of tandem repeats upstream of the insulin gene is a susceptibility locus for latent autoimmune diabetes in adults. Diabetes 55:1890–1894
Bottini N, Vang T, Cucca F, Mustelin T (2006) Role of PTPN22 in type 1 diabetes and other autoimmune diseases. Semin Immunol 18:207–213
Onengut-Gumuscu S, Buckner JH, Concannon P (2006) A haplotype-based analysis of the PTPN22 locus in type 1 diabetes. Diabetes 55:2883–2889
Helgason A, Palsson S, Thorleifsson G et al (2007) Refining the impact of TCF7L2 gene variants on type 2 diabetes and adaptive evolution. Nat Genet 39:218–225
Grant SF, Thorleifsson G, Reynisdottir I et al (2006) Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 38:320–323
Bell GI, Horita S, Karam JH (1984) A polymorphic locus near the human insulin gene is associated with insulin-dependent diabetes mellitus. Diabetes 33:176–183
Hitman GA, Tarn AC, Winter RM et al (1985) Type 1 (insulin-dependent) diabetes and a highly variable locus close to the insulin gene on chromosome 11. Diabetologia 28:218–222
Thomson G, Robinson WP, Kuhner MK, Joe S, Klitz W (1989) HLA and insulin gene associations with IDDM. Genet Epidemiol 6:155–160
Lucassen AM, Julier C, Beressi JP et al (1993) Susceptibility to insulin dependent diabetes mellitus maps to a 4.1 kb segment of DNA spanning the insulin gene and associated VNTR. Nat Genet 4:305–310
Reinehr T, Schober E, Wiegand S, Thon A, Holl R (2006) Beta-cell autoantibodies in children with type 2 diabetes mellitus: subgroup or misclassification? Arch Dis Child 91:473–477
Haller K, Kisand K, Pisarev H et al (2007) Insulin gene VNTR, CTLA-4+49A/G and HLA-DQB1 alleles distinguish latent autoimmune diabetes in adults from type 1 diabetes and from type 2 diabetes group. Tissue Antigens 69:121–127
Hosszufalusi N, Vatay A, Rajczy K et al (2003) Similar genetic features and different islet cell autoantibody pattern of latent autoimmune diabetes in adults (LADA) compared with adult-onset type 1 diabetes with rapid progression. Diabetes Care 26:452–457
Saxena R, Voight BF, Lyssenko V et al (2007) Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316:1331–1336
Scott LJ, Mohlke KL, Bonnycastle LL et al (2007) A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316:1341–1345
Sladek R, Rocheleau G, Rung J et al (2007) A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885
Zeggini E, Weedon MN, Lindgren CM et al (2007) Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316:1336–1341
Bennett ST, Todd JA (1996) Human type 1 diabetes and the insulin gene: principles of mapping polygenes. Annu Rev Genet 30:343–370
Ong KK, Phillips DI, Fall C et al (1999) The insulin gene VNTR, type 2 diabetes and birth weight. Nat Genet 21:262–263
Yi F, Brubaker PL, Jin T (2005) TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J Biol Chem 280:1457–1464
Morin PJ, Sparks AB, Korinek V et al (1997) Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275:1787–1790
Acknowledgements
We thank all Swedish diabetologists and diabetes nurses who contributed to DISS. M. Svenson, A. Berglund, E. Laurila, M. Svensson, C. Rosborn, U. Gustavsson, A. Radelius, G. Gremsperger and J. Pilz are acknowledged for expert technical assistance. The Juvenile Diabetes Foundation Wallenberg Diabetes Research Program (K 98-99 JD-128 13), the Swedish Diabetes Association, the Swedish Medical Research Council (72X-14531), the Albert Påhlsson Foundation, Research Fund at Malmö University Hospital are acknowledged for support of DISS and Knut & Alice Wallenberg foundation for equipment availability.
Duality of interest
L. Groop has been a consultant for and served on advisory boards for Lilly, Sanofi-Aventis, GSK, Novartis and Tethys Bioscience. All other authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
G. Sundkvist died in September 2006.
Electronic supplementary material
Below is the link to the supplementary material
ESM Table 1
Combination of TCF7L2 genotypes (rs7903146 and rs1088540) in young diabetic patients (15–34 years) and healthy controls (PDF 19.8 KB)
ESM Table 2
Clinical characteristics of young (15–34 years at onset) diabetic patients with and without islet auto-antibodies (PDF 110 KB)
ESM Table 3
Clinical characteristics of young (15–34 years at onset) GADA-positive vs GADA-negative diabetic patients (PDF 74.9 KB)
ESM Table 4
Prevalence of HLA-DQB1, PTPN22, INS VNTR and TCF7L2 in young (15–34 years at onset) GADA-positive vs GADA-negative diabetic patients (PDF 85.5 KB)
ESM Table 5
Prevalence of HLA-DQB1, PTPN22, INS VNTR and TCF7L2 genotypes in young (15–34 years at onset) GADA-positive vs GADA-negative diabetic patients stratified for median GADA values (PDF 32.4 KB)
ESM Table 6
Prevalence of TCF7L2 genotypes among young (15–34 years old), middle-aged (40–59 years old) diabetic patients and non-diabetic controls (PDF 65.3 KB)
ESM Table 7
Prevalence of HLA-DQB1, PTPN22, INS VNTR and TCF7L2 genotypes in middle-aged (40–59 years at onset) GADA-positive vs GADA-negative diabetic patients stratified for median GADA values (PDF 32.2 KB)
ESM Table 8
Prevalence of HLA-DQB1, PTPN22, INS VNTR, TCF7L2 (rs7903146 and rs10885406) in GADA-positive vs GADA-negative diabetic patients stratified for age at onset of diabetes between 15–25 and 25–34 years (PDF 86.6 KB)
Rights and permissions
About this article
Cite this article
Bakhtadze, E., Cervin, C., Lindholm, E. et al. Common variants in the TCF7L2 gene help to differentiate autoimmune from non-autoimmune diabetes in young (15–34 years) but not in middle-aged (40–59 years) diabetic patients. Diabetologia 51, 2224–2232 (2008). https://doi.org/10.1007/s00125-008-1161-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-008-1161-2