Abstract
Aims/hypothesis
Minimal model analysis for insulin sensitivity has been validated against the glucose clamp and is an accepted method for estimating insulin sensitivity from IVGTT. However minimal model analysis requires a 3 h test and relevant expertise to run the mathematical model. The aim of this study was to suggest a simple predictor of minimal model analysis index using only 1 h IVGTT.
Methods
We studied participants with different clinical characteristics who underwent 3 h regular (n = 336) or insulin-modified (n = 160) IVGTT, or 1 h IVGTT and euglycaemic–hyperinsulinaemic clamp (n = 247). Measures of insulin sensitivity were insulin sensitivity index estimated by minimal model analysis (SI) and the mean glucose infusion rate (clamp) (M). A calculated SI (CSI) predictor, \({\text{CS}}_{{\text{I}}} = {\text{ $ \alpha $ }} \times {K_{\text G} } \mathord{\left/ {\vphantom {{K_{G} } {{\left( {{\Delta {\text{AUC}}_{{{\text{INS}}}} } \mathord{\left/ {\vphantom {{\Delta {\text{AUC}}_{{{\text{INS}}}} } T}} \right. \kern-\nulldelimiterspace} T} \right)}}}} \right. \kern-\nulldelimiterspace} {{\left( {{\Delta {\text{AUC}}_{{{\text{INS}}}} } \mathord{\left/ {\vphantom {{\Delta {\text{AUC}}_{{{\text{INS}}}} } T}} \right. \kern-\nulldelimiterspace} T} \right)}}\), was suggested, based on the calculation of the rate of glucose disappearance K G and the suprabasal AUC of insulin concentration ΔAUCINS over T = 40 min. For all the participants, α was assumed equal to the regression line slope between K G/(ΔAUCINS/T) and SI in control participants.
Results
CSI and SI showed high correlation (R 2 = 0.68–0.96) and regression line slopes of approximately one in the majority of groups. CSI tended to overestimate SI in type 2 diabetic participants, but results were more reliable when CSI was computed with insulin-modified rather than regular IVGTT. CSI showed behaviours similar to SI as regards relationships with BMI, acute insulin response and sex. CSI showed good correlation with M (R 2 = 0.82).
Conclusions/interpretation
A short test can achieve a good approximation of minimal model analysis and clamp insulin sensitivity. The importance of a method such as CSI is that it allows analysis of IVGTT datasets with samples limited to 1 h.
Similar content being viewed by others
Introduction
Insulin sensitivity is paramount for characterising metabolic states. The glucose clamp is the experimental procedure yielding the gold standard measurement of this variable. Nonetheless, minimal model analysis of IVGTT data, i.e. insulin sensitivity index estimated by minimal model analysis (SI), is also widely used to assess insulin sensitivity [1, 2]. However, despite some efforts to develop automatic procedures and lower the need for user intervention [3], the minimal model procedure requires sophisticated computer programming and relevant expertise to run the mathematical model properly. Furthermore, reliable results based on minimal model require many plasma insulin and glucose samples over a time interval of at least 3 h after glucose injection.
The aims of this study were: (1) to propose an index able to predict minimal model insulin sensitivity values based on direct calculations from easily measurable simple variables and not requiring complex mathematical models, while using IVGTT data limited to 1 h or less, as often happened before introduction of the minimal model [4]; and (2) to validate the new index against minimal model SI and the glucose clamp, by assessing its performance in several groups of participants with different degree of glucose tolerance and specific clinical characteristics.
Methods
Participants analysed in this study are presented in the following sections. All participants gave their consent to the investigations, which were approved by the Local Ethics Committees.
Participants, 3 h regular IVGTT
We analysed 336 participants partially studied in previous investigations [5–8]. Of these, 114 were control participants with normal glucose tolerance (NGT), 128 had impaired glucose tolerance (IGT) (22 of whom also had impaired fasting glucose) and 22 had type 2 diabetes (Table 1). The type 2 diabetes patients (diabetes duration 6.2 ± 0.4 years) were diet-controlled; none of them were taking oral hypoglycaemic agents or insulin. We also analysed 52 participants with chronic renal disease from diabetic nephropathy, nine patients with hyperparathyroidism before and after parathyroidectomy, and 11 patients who previously had type 1 diabetes (prior to kidney–pancreas transplantation) (Table 1). All participants underwent a regular 3 h frequently sampled IVGTT [9].
Type 2 diabetic participants, 3 h insulin-modified IVGTT
We analysed from previous studies [10–12] 160 type 2 diabetic participants who had undergone an insulin-modified, 3 h, frequently sampled IVGTT (INSMOD) with exogenous intravenous infusion of insulin (0.03 or 0.05 U/kg) at 20 min [9] (Table 1). Some of these participants were under pharmacological treatment, with gemfibrozil [10], sulfonylurea or biguanide preparations [11].
Participants, 1 h IVGTT and clamp
We analysed 247 participants from the Botnia study [13], the EUGENE2 study [14] and another study [15]. All these participants underwent IVGTT (for at least 1 h) and 2 h euglycaemic–hyperinsulinaemic glucose clamp. Among participants undergoing the clamp, 171 had NGT (NGTCL), 55 had impaired glucose metabolism (IGMCL), i.e. either impaired fasting glucose or IGT or both, and 21 had type 2 diabetes (Table 1). Seven participants in the type 2 diabetes clamp group had severe obesity and subsequently underwent bariatric surgery (here we only report data before surgery).
Calculation of insulin sensitivity
In the participants with regular and INSMOD data, insulin sensitivity index was estimated by minimal model analysis (SI). In the participants with the clamp, insulin sensitivity was calculated as the mean glucose infusion rate (M) over the last 40 min of the test. For all participants, we calculated a surrogate index of SI, called calculated SI (CSI), with an expression similar to that originally proposed by Galvin et al. [16]. Justification of the difference between our approach and that of Galvin et al. [16] is discussed later. For the participants with regular IVGTT the expression for CSI was:
where α is a constant (scaling factor), K G is the rate of glucose disappearance (slope of log glucose), ΔAUCINS is the AUC of insulin concentration above basal value and T is the time interval between 10 and 50 min (=40 min) when K G and ΔAUCINS are computed. Initial time interval was not zero to avoid possible confounding effects due to mixing. The α constant was assumed equal to the slope of the regression line between the factor K G/(ΔAUCINS/T) and SI in the control group, i.e. α = 0.276. This value was used to calculate CSI in all the participants analysed in this study, including those undergoing INSMOD rather than regular IVGTT or clamp.
For the participants with INSMOD the expression for CSI was:
It is well known that the action of exogenous insulin on glucose disappearance is delayed [17], and hence we assumed a 5 min delay. Since insulin was injected at 20 min, K G1 and ΔAUCINS1 were computed between 10 and 25 min, whereas K G2 and ΔAUCINS2 were computed between 25 and 50 min.
Statistical analysis
Relationships between SI and CSI were investigated by linear regression analysis with no intercept. Difference between the mean value of SI and CSI in each of the different groups of participants was assessed through the paired t test. The same test was used to assess difference in insulin sensitivity in the hyperparathyroidism group before and after surgery. Difference in the mean value of each index among different groups was assessed through ANOVA. Similarly, we analysed the relationship between CSI and M by linear regression and used ANOVA to assess differences of both indices among different groups. Relationships between some variables were also investigated by accounting for measurement errors for both variables in the regression [18]. Normality of distributions was assessed before testing for possible differences in insulin sensitivity indices. In case of non-normal distributions, tests were performed on logarithmically transformed values (this applied to the majority of cases, except hyperparathyroidism and former type 1 diabetes groups). p < 0.05 was considered statistically significant. Values are reported as mean ± SE.
Results
Minimal model and CSI analyses of regular IVGTT
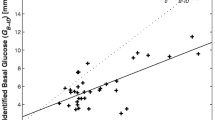
Strong correlation between SI and CSI was found in the following groups: control (R 2 = 0.89, p < 0.0001, slope = 1.00, 95% CI 0.93–1.07), IGT (R 2 = 0.79, p < 0.0001, slope = 0.97, 95% CI 0.89–1.06), renal disease (R 2 = 0.85, p < 0.0001, slope = 0.86, 95% CI 0.76-0.97), former type 1 diabetes patients (after kidney pancreas transplantation) (R 2 = 0.89, p < 0.0001, slope = 0.75, 95% CI 0.57-0.93) and hyperparathyroidism (R 2 = 0.83, p < 0.0001, slope = 1.09, 95% CI 0.68-1.49 before surgery; R 2 = 0.96, p < 0.0001, slope = 1.24, 95% CI 1.02–1.46 after surgery) (Fig. 1). In the type 2 diabetes group the correlation between SI and CSI, though weaker than in the other groups, was still significant (R 2 = 0.68, p < 0.0001), despite the fact that CSI overestimated SI (slope = 1.75, 95% CI 1.21-2.29). When the participants were considered all together, the correlation remained highly significant (R 2 = 0.84, p < 0.0001, slope = 0.99, 95% CI 0.94-1.04).
Regression plots between insulin sensitivity from minimal model (SI) and from CSI index in (a) control participants, (b) participants with IGT, (c) type 2 diabetic participants (d) participants with chronic renal disease, (e) participants with hyperparathyroidism before and after parathyroidectomy and (f) former type 1 diabetic patients after kidney–pancreas transplantation. e White circles and dotted line are related to the condition after surgery. To convert values for SI to SI units (× 10−4 min−1 [pmol/l]−1), multiply by 0.1667
In each group, mean values of SI and CSI (Table 1) were not different except for the type 2 diabetes and hyperparathyroidism after surgery groups, which showed a slight difference as shown by p values (Table 1). Bland–Altman plot for all the participants (Fig. 2a) proved substantial equivalence between the two measurements. The absolute difference between SI and CSI in relation to the AUC of insulin in the time interval T (Fig. 2b) showed that only at low insulin levels did SI and CSI tend to diverge. ANOVA showed that SI was different between control and all the other groups (p < 0.03) except for the hyperparathyroidism after surgery group. Significant differences were also found in the renal disease vs IGT and type 2 diabetes groups (p < 0.0001), and in the hyperparathyroidism after surgery vs IGT and type 2 diabetes groups (p < 0.0006). Similar results were found for CSI (p value range: p < 0.0001 to p = 0.0313), except for comparisons of type 2 diabetes with the other groups. It is worth noting the difference in insulin sensitivity between hyperparathyroidism before and after surgery: as expected, SI was increased after surgery (p = 0.021) (Table 1) and similar results were found with CSI (p = 0.008).
Bland–Altman plot for SI and CSI in all the participants that underwent regular IVGTT (a) and absolute difference between SI and CSI in relation to the insulin AUC (b). Dotted lines (a) represent mean (middle line), 1.96 SD (top) and −1.96 SD (bottom). To convert values for SI and CSI to SI units (×10−4 min−1 [pmol/l]−1), multiply by 0.1667
In the control group, we calculated the relationships between insulin sensitivity and acute insulin response to glucose (AIRG) (mean insulin value above basal in the 0 to 8 min period of the IVGTT). Both SI and CSI showed with AIRG a weak but significant nonlinear inverse relationship, which was better appreciated after performing linear regression analysis on logarithmically transformed values (R 2 = 0.19, p = 0.0002 for SI; R 2 = 0.09, p = 0.0009 for CSI) (Fig. 3). According to ordinary least-squares regression analysis, the relationship was not strictly hyperbolic, but it was similar with both indices. However, when the analysis was carried out through a regression method accounting for measurement errors in both variables, the relationship turned out to be hyperbolic, as the 95% CI for the slope included −1 for SI (slope: −1.33, 95% CI −2.08, −0.59) and CSI (slope: −1.25, 95% CI −2.15, −0.35).
Regression plot between SI and AIRG (a), and CSI and AIRG (b) in the control participants that underwent regular IVGTT. Variables are logarithmically transformed (log e ). Regression line equations were: for \( {\text{S}}_{\text{I}} \,y = 1.98 - 0.46x \) (95% CI for intercept and slope 0.19, 3.77 and −0.69, −0.22, respectively); for \( {\text{CS}}_{\text{I}} \,y = 2.80 - 0.35x \) (95% CI for intercept and slope 1.24, 4.36 and −0.56, −0.15, respectively). To convert values for SI and CSI to SI units (× 10−4 min−1 [pmol/l]−1), multiply by 0.1667
In all the participants, we also analysed insulin sensitivity with respect to BMI. As expected, SI showed an inverse relationship with BMI; in fact, after log-log transformation, a weak but significant linear regression was observed (R 2 = 0.19, p < 0.0001), although the relationship was not hyperbolic (according to both regression methods). Similar results were found for CSI (R 2 = 0.18, p < 0.0001). Participants were then classified as lean or overweight according to their BMI (threshold 25 kg/m2). Both SI and CSI showed significant differences in insulin sensitivity between the two groups (SI = 4.65 ± 0.32 × 10−4 min−1 [μU/ml]−1 lean; 3.09 ± 0.28 overweight; p = 0.0003; CSI = 5.03 ± 0.38 × 10−4 min−1 [μU/ml]−1 lean; 2.99 ± 0.28 overweight; p < 0.0001; to convert values for SI and CSI to SI units (× 10−4 min−1 [pmol/l]−1), multiply by 0.1667).
We also studied possible differences in insulin sensitivity due to sex: neither SI nor CSI were different: SI = 3.87 ± 0.17 × 10−4 min−1 (μU/ml)−1 men; 4.09 ± 0.28 women; p > 0.4; CSI = 4.11 ± 0.20 × 10−4 min−1 (μU/ml)−1 men; 4.77 ± 0.33 women; p > 0.07.
Minimal model and CSI analyses of insulin-modified IVGTT
In the type 2 diabetes INSMOD group, SI and CSI showed strong significant correlation, with the slope of the regression line virtually equal to 1 (R 2 = 0.85, p < 0.0001, slope = 0.96, 95% CI 0.89-1.02) (Fig. 4a). Bland–Altman plot showed that only a few samples were outside the limits for equivalence (Fig. 4b). The paired t test showed a borderline p value (Table 1). We also classified the participants as obese or non-obese. Since BMI was quite high on average (BMI = 29.7 ± 0.4 kg/m2), we assumed 27.5 as threshold between the two groups. As expected, SI was higher in the non-obese group, with similar results found for CSI (SI = 1.77 ± 0.18 × 10−4 min−1 [μU/ml]−1 non-obese; 0.94 ± 0.08 obese; p < 0.0001; CSI = 1.73 ± 0.17 × 10−4 min−1 [μU/ml]−1 non-obese; 1.10 ± 0.08 obese; p = 0.0002). Neither SI nor CSI were different between men and women (SI = 1.19 ± 0.11 × 10−4 min−1 [μU/ml]−1 men; 1.04 ± 0.14 women; p > 0.5; CSI = 1.30 ± 0.10 × 10−4 min−1 [μU/ml]−1 men; 1.03 ± 0.11 women; p > 0.2).
Regression plot between SI and CSI in type 2 diabetic participants that underwent INSMOD (a) and Bland–Altman plot for SI and CSI (b). Dotted lines (b) represent mean (middle line), 1.96 SD (top) and −1.96 SD (bottom). To convert values for SI and CSI to SI units (× 10−4 min−1min−1 [pmol/l]−1), multiply by 0.1667
Glucose clamp and CSI analyses
In all the participants grouped together, M and CSI showed good correlation (R 2 = 0.82, p < 0.0001) (Fig. 5a). When analysing the participants divided according to glucose tolerance, correlation remained significant (NGTCL: R 2 = 0.84, p < 0.0001; IGMCL: R 2 = 0.74, p < 0.0001; type 2 diabetes clamp: R 2 = 0.81, p < 0.0001) (Fig. 5b). M (Table 1) was different in each group (p < 0.03). Similar differences (p < 0.002) were found with CSI (Table 1) except in the IGMCL and type 2 diabetes clamp groups, where statistical significance was not reached. M was higher in lean than in overweight participants (8.72 ± 0.33 and 5.58 ± 0.18 mg min−1 kg−1, p < 0.0001; to convert values for M to SI units [mmol min−1 kg−1], multiply by 0.005551), as was the case for CSI (7.56 ± 0.38 and 4.26 ± 0.20 × 10−4 min−1 [μU/ml]−1, p < 0.0001). As regards possible differences related to sex, neither M nor CSI showed any difference (p > 0.07). In a subgroup of participants, we corrected M for the steady-state insulin level, but results did not change significantly (not shown). It is worth noting that in the small group of type 2 diabetes clamp participants with severe obesity, 3 h IVGTT data were available, thus SI was computed. As expected, we found agreement between SI and M, with regression coefficient value (R 2 = 0.63, p = 0.018) similar to those observed between CSI and M, as reported above. In this specific subgroup, CSI showed a very strong relationship with M (R 2 = 0.95, p < 0.0001).
Regression plot between insulin sensitivity from the clamp (M) and from the empirical index (CSI) in (a) NGTCL participants (circles), IGMCL participants (squares) and type 2 diabetes clamp group participants (triangles). The regression line equation was \( y = 7.82 \times 10^{{ - 5}} x \) (95% CI 7.36, 8.27 × 10−5). Regression plot as above (a) for type 2 diabetes clamp group alone (b). Regression line equation: \( y = 8.08 \times 10^{{ - 5}} x \) (95% CI 6.27, 9.89 × 10−5). To convert values for M to SI units (mmol min−1 kg−1), multiply by 0.005551
Discussion
The simple index of insulin sensitivity introduced and validated here (CSI) was revealed to be a good surrogate of that from the well accepted and widely used minimal model (SI). To our knowledge, only the study of Galvin et al. [16] suggested a simple index for the assessment of insulin sensitivity from IVGTT limited to 1 h. CSI reflects similar concepts, i.e. the quantification of glucose disappearance rate per changes of insulin, but it overcomes some limitations of that study. In fact, Galvin et al. [16] studied the correlation of their index with SI (and also with insulin sensitivity by the glucose clamp), but they did not seek to obtain indices really comparable, their units being different. In addition, they did not present any strategy to correct their index and make it comparable with SI derived from insulin-modified IVGTT. In contrast, CSI includes a time (T) factor (see Eq. 1) yielding the same units as SI and was adapted to be used also with the insulin-modified test (Eq. 2). Furthermore, in Galvin et al. [16], the slopes of the regression lines were far from one and different in every group. Moreover, only small groups of participants were studied (with no diabetic patients) and it was not shown whether their index has abilities, similar to SI, to discriminate between groups or clinical conditions with different degrees of insulin resistance. The Galvin index [16] was then used by Anderson et al. [19], but with essentially the same limitations, which probably prevented its diffusion. Prior to this study, we used calculations similar to those for CSI to compute a sensitivity index in mice [20], although not with exactly the same formula and without comparison with the clamp.
After correcting our index with a factor derived from regression analysis of the control group (quite a large group, with a wide range of insulin sensitivity), several other groups of participants with different degrees of glucose tolerance and heterogeneous clinical characteristics were analysed. In the majority of groups, we found a good correlation between SI and CSI, and also CSI values similar to SI, as mirrored both by the slope of the regression lines, which were not (or only slightly) different from 1 (see 95% CI), and by the not significantly different mean values.
The correction factor α included in the CSI expression was introduced to scale the values of our new index to those calculated with the minimal model. Thus, the interpretation of results obtained by CSI will be facilitated, given the previous wide experience with SI. This correction factor does not have a specific physiological meaning, similarly to the variables included in other empirical methods for the calculation of insulin sensitivity, such as HOMA-insulin resistance (IR) [21] or Stumvoll’s index [22]. The relevant aspect of the scaling operation was that the same value of the correction factor (α = 0.276) was proved to be appropriate in every group of participants (except type 2 diabetes, as discussed below). In fact, all the results were obtained by using the same correction factor in each group that underwent the regular IVGTT. The same α value was also proved correct in those groups of participants who underwent INSMOD (type 2 diabetes INSMOD) or the clamp (NGTCL, IGMCL, type 2 diabetes clamp).
The comparison between SI and CSI was not completely satisfactory in type 2 diabetes (regular IVGTT). The fact that in situations of high insulin resistance CSI tended to overestimate SI is an important issue and should be discussed within the frame of basic questions, such as: how reliable is a low SI? This has been much debated among investigators using IVGTT [23, 24]. Thus, we acknowledge that, in situations of low insulin sensitivity, CSI may suffer from inaccuracy, but SI may also exhibit inaccuracy in those conditions [24, 25]. As regards our data, insulin levels in the type 2 diabetes group were usually low, but tended to remain higher than the fasting value: i.e. insulin levels did not return to the basal value during the whole 3 h IVGTT time interval. Thus, in the minimal model approach, the analysis of the last part of the IVGTT tended to decrease the SI value. Since the last part of the complete test is not accounted for by CSI, some discrepancy between the two indices may occur. On the other hand, the finding that in the majority of groups CSI behaves similarly to SI suggests that the information provided by the last part of the IVGTT is usually consistent with that provided by the first part, where CSI is calculated.
Due to the unsatisfactory results in the type 2 diabetes group, we adapted the CSI expression to make it usable with data from the insulin-modified IVGTT as recommended in conditions of poor insulin response [26]. We analysed a large group of type 2 diabetic patients subjected to INSMOD where, as expected, CSI and SI showed low values of insulin sensitivity. They also exhibited a strong correlation with regression slope almost identical with 1, confirming that when dealing with low insulin sensitivity it is recommended to carry out the insulin-modified test even with the short 1 h protocol. We also analysed 208 insulin-modified IVGTT from 146 women with a history of gestational diabetes, who were non-diabetic at the time of examination [27]. We found strong relationship between SI and CSI, with R 2 = 0.93 and slope of the regression almost equal to 1 (not shown). However, in non-diabetic participants the regular IVGTT has proven adequate for calculating CSI with sufficient accuracy; hence the insulin-modified protocol is not strictly necessary in these participants. It should be noted that other possible expressions were tested for the calculation of CSI with the insulin-modified IVGTT, such as the average between \({{\left( {{\text{ $ \alpha $ }} \times K_{{{\text{G1}}}} } \right)}} \mathord{\left/ {\vphantom {{{\left( {{\text{ $ \alpha $ }} \times K_{{{\text{G1}}}} } \right)}} {{\left( {{\Delta {\text{AUC}}_{{{\text{INS1}}}} } \mathord{\left/ {\vphantom {{\Delta {\text{AUC}}_{{{\text{INS1}}}} } T}} \right. \kern-\nulldelimiterspace} T1} \right)}}}} \right. \kern-\nulldelimiterspace} {{\left( {{\Delta {\text{AUC}}_{{{\text{INS1}}}} } \mathord{\left/ {\vphantom {{\Delta {\text{AUC}}_{{{\text{INS1}}}} } T}} \right. \kern-\nulldelimiterspace} T1} \right)}}\) and \({{\left( {{\text{ $ \alpha $ }} \times K_{{{\text{G2}}}} } \right)}} \mathord{\left/ {\vphantom {{{\left( {{\text{ $ \alpha $ }} \times K_{{{\text{G2}}}} } \right)}} {{\left( {{\Delta {\text{AUC}}_{{{\text{INS2}}}} } \mathord{\left/ {\vphantom {{\Delta {\text{AUC}}_{{{\text{INS2}}}} } T}} \right. \kern-\nulldelimiterspace} T2} \right)}}}} \right. \kern-\nulldelimiterspace} {{\left( {{\Delta {\text{AUC}}_{{{\text{INS2}}}} } \mathord{\left/ {\vphantom {{\Delta {\text{AUC}}_{{{\text{INS2}}}} } T}} \right. \kern-\nulldelimiterspace} T2} \right)}}\), with T1 = 15 and T2 = 25 min, and also the second expression alone (i.e. only post-injection information). However, the best results in diabetic and non-diabetic participants were obtained by combining pre- and post-injection information as in Equation (2).
CSI was able to reproduce known findings related to insulin sensitivity. The existence of nonlinear inverse (hyperbolic) relationship between insulin sensitivity and insulin release was postulated some years ago [28] and several subsequent studies [29] have confirmed this finding, although it has recently been suggested that the hyperbola may not be evident in some groups of participants [30–32]. Our control group exhibited a weak, but still significant inverse relationship between insulin sensitivity and AIRG. According to traditional regression analysis, the relationship was not strictly hyperbolic, but when a more refined regression model was used the hyperbolic relationship emerged. It is worth noting that SI and CSI provided similar results in both cases. Insulin sensitivity was higher in lean than in overweight or obese participants with both indices, which also showed a nonlinear inverse relationship (though weak) with BMI, in agreement with previous studies [33]. As regards the effect of sex on insulin sensitivity, results from SI and CSI were again similar and in agreement with previous studies [34].
Even though a good agreement was found between SI and CSI, we aimed to validate CSI against the measurement obtained from the glucose clamp. CSI exhibited a good degree of correlation with M and a similar ability to discriminate between participants with different glucose tolerance, as well as between lean and overweight participants. This agreement with the clamp further strengthened the ability of CSI to describe insulin sensitivity in different metabolic conditions.
In this study we included three groups of type 2 diabetic patients. As regards the type 2 diabetes and type 2 diabetes INSMOD groups, it must be noted (Table 1) that both SI and CSI were higher in the former than the latter (p < 0.0001 by ANOVA). This possible inconsistency warrants further comment. First, it cannot be excluded that this difference in insulin sensitivity was real, since type 2 diabetic populations may be significantly heterogeneous [35]. On the other hand, as already pointed out, SI may be inaccurate in participants with low insulin values, and CSI exhibits similar limitations in those conditions. Another confounding factor may be the fact that the type 2 diabetes and type 2 diabetes INSMOD groups were studied in different laboratories, probably using different insulin assays: this remains a problem known to be a possible source of error [36]. In any case, we believe that the lower insulin sensitivity in the type 2 diabetes INSMOD than in the type 2 diabetes group may not be an artefact: in fact, HOMA-IR was also clearly higher in the former (7.85 vs 3.47 [non-dimensional], p < 0.007), possibly also due the much higher BMI (Table 1). Similar comments hold for the significant difference in CSI values (p < 0.0001) between IGT and IGMCL.
In conclusion, although the minimal model analysis remains the reference method to assess insulin sensitivity from the 3 h IVGTT, the proposed simple, empirical index CSI generally proved to be a reliable index. In the condition of low insulin sensitivity, quite common in type 2 diabetes, analysis of insulin-modified rather than regular IVGTT data should be performed to obtain more reliable estimations, although it is known that in such conditions the assessment of insulin sensitivity becomes intrinsically more uncertain and possibly inaccurate. The great advantage of CSI is that it allows assessment of insulin sensitivity from IVGTT data limited to 1 h, which cannot be analysed with the minimal model. The possibility of analysing less expensive short IVGTTs makes performance of the test easier and less of a burden for participants and investigators, allowing in larger populations the simultaneous assessment of insulin sensitivity and beta cell function (e.g. AIRG variable) with a simple approach. CSI also allows retrospective studies on all the short IVGTTs commonly performed before the introduction of the minimal model.
Abbreviations
- AIRG :
-
Acute insulin response to glucose
- AUCINS :
-
AUC of insulin concentration
- CSI :
-
Calculated SI
- IGMCL :
-
Impaired glucose metabolism (participants subjected to clamp)
- IGT:
-
Impaired glucose tolerance
- INSMOD:
-
Insulin-modified 3 h frequently sampled IVGTT
- M :
-
Mean glucose infusion rate (clamp)
- NGT:
-
Normal glucose tolerance
- NGTCL :
-
NGT participants subjected to clamp
- SI :
-
Insulin sensitivity index estimated by minimal model analysis
References
Bergman RN (1989) Toward physiological understanding of glucose tolerance. Minimal model approach. Diabetes 38:1512–1527
Saad MF, Anderson RL, Laws A et al (1994) A comparison between the minimal model and the glucose clamp in the assessment of insulin sensitivity across the spectrum of glucose tolerance. Diabetes 43:1114–1121
Morbiducci U, Di Benedetto G, Kautzky-Willer A, Pacini G, Tura A (2007) Improved usability of the minimal model of insulin sensitivity based on an automated approach and genetic algorithms for parameter estimation. Clin Sci (Lond) 112:257–263
Amatuzio DS, Stutzman FL, Vanderbilt MJ, Nesbitt S (1953) Interpretation of the rapid intravenous glucose tolerance test in normal individuals and in mild diabetes mellitus. J Clin Invest 32:428–435
Viviani GL, Pacini G (1999) Reduced glucose effectiveness as a feature of glucose intolerance: evidence in elderly type-2 diabetic subjects. Aging 11:169–175
Fliser D, Pacini G, Engelleiter R et al (1998) Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int 53:1343–1347
Kautzky-Willer A, Pacini G, Niederle B, Schernthaner G, Prager R (1992) Insulin secretion, insulin sensitivity and hepatic insulin extraction in primary hyperparathyroidism before and after surgery. Clin Endocrinol (Oxf) 37:147–155
Rigotti P, Pacini G, Baldan N et al (1998) Insulin secretion in IDDM patients who have undergone successful pancreas–kidney transplantation. Transplant Proc 30:615–617
Pacini G, Tonolo G, Sambataro et al (1998) Insulin sensitivity and glucose effectiveness: minimal model analysis of regular and insulin-modified IVGTT. Am J Physiol Endocrinol Metab 274:E592–E599
Avogaro A, Miola M, Favaro A et al (2001) Gemfibrozil improves insulin sensitivity and flow-mediated vasodilatation in type 2 diabetic patients. Eur J Clin Invest 31:603–609
Avogaro A, Watanabe RM, Dall’Arche A, de Kreutzenberg SV, Tiengo A, Pacini G (2004) Acute alcohol consumption improves insulin action without affecting insulin secretion in type 2 diabetic subjects. Diabetes Care 27:1369–1374
Ludvik B, Hanefeld M, Pacini G (2008) Improved metabolic control by Ipomoea batatas (Caiapo) is associated with increased adiponectin and decreased fibrinogen levels in type 2 diabetic subjects. Diabetes Obes Metab 10:586–592
Groop L, Forsblom C, Lehtovirta M et al (1996) Metabolic consequences of a family history of NIDDM (the Botnia study): evidence for sex-specific parental effects. Diabetes 45:1585–1593
Laakso M, Zilinskaite J, Hansen T et al (2008) EUGENE2 consortium: insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia 51:502–511
Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G (2009) First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care 32:375–380
Galvin P, Ward G, Walters J et al (1992) A simple method for quantitation of insulin sensitivity and insulin release from an intravenous glucose tolerance test. Diabet Med 9:921–928
Sherwin RS, Kramer KJ, Tobin JD et al (1974) A model of the kinetics of insulin in man. J Clin Invest 53:1481–1492
Combleet PJ, Gochman N (1979) Incorrect least-squares regression coefficients in method-comparison analysis. Clin Chem 25:432–438
Anderson RL, Hamman RF, Savage PJ et al (1995) Exploration of simple insulin sensitivity measures derived from frequently sampled intravenous glucose tolerance (FSIGT) tests. The Insulin Resistance Atherosclerosis Study. Am J Epidemiol 142:724–732
Pacini G, Ahrén M, Ahren B (2009) Reappraisal of the intravenous glucose tolerance index for a simple assessment of insulin sensitivity in mice. Am J Physiol Regul Integr Comp Physiol 296:R1316–R1324
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Stumvoll M, Mitrakou A, Pimenta W et al (2000) Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 23:295–301
Haffner SM, D’Agostino R Jr, Festa A et al (2003) Low insulin sensitivity (S(i) = 0) in diabetic and nondiabetic subjects in the insulin resistance atherosclerosis study: is it associated with components of the metabolic syndrome and nontraditional risk factors? Diabetes Care 26:2796–2803
Mari A (1997) Assessment of insulin sensitivity with minimal model: role of model assumptions. Am J Physiol Endocrinol Metab 272:E925–E934
Ferrannini E, Mari A (1998) How to measure insulin sensitivity. J Hypertens 16:895–906
Finegood DT, Hramiak IM, Dupre J (1990) A modified protocol for estimation of insulin sensitivity with the minimal model of glucose kinetics in patients with insulin-dependent diabetes. J Clin Endocrinol Metab 70:1538–1549
Kautzky-Willer A, Krssak M, Winzer C et al (2003) Increased intramyocellular lipid concentration identifies impaired glucose metabolism in women with previous gestational diabetes. Diabetes 52:244–251
Bergman RN, Phillips LS, Cobelli C (1981) Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68:1456–1467
Kahn SE, Prigeon RL, McCulloch DK et al (1993) Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42:1663–1672
Ferrannini E, Mari A (2004) Beta cell function and its relation to insulin action in humans: a critical appraisal. Diabetologia 47:943–956
Pacini G (2006) The hyperbolic equilibrium between insulin sensitivity and secretion. Nutr Metab Cardiovasc Dis 16(Suppl 1):S22–S27
Tura A, Mari A, Prikoszovich T, Pacini G, Kautzky-Willer A (2008) Value of the intravenous and oral glucose tolerance tests for detecting subtle impairments in insulin sensitivity and beta-cell function in former gestational diabetes. Clin Endocrinol (Oxf) 69:237–243
Garca-Estévez DA, Araújo-Vilar D, Saavedra-González A, Fiestras-Janeiro G, Cabezas-Cerrato J (2004) Analysis of the relationship between body mass index, insulin resistance, and beta-cell function: a cross-sectional study using the minimal model. Metabolism 53:1462–1466
Clausen JO, Borch-Johnsen K, Ibsen H et al (1996) Insulin sensitivity index, acute insulin response, and glucose effectiveness in a population-based sample of 380 young healthy Caucasians. Analysis of the impact of gender, body fat, physical fitness, and life-style factors. J Clin Invest 98:1195–1209
Zhang Y, Wat N, Stratton IM et al (1996) UKPDS 19: heterogeneity in NIDDM: separate contributions of IRS-1 and beta 3-adrenergic-receptor mutations to insulin resistance and obesity respectively with no evidence for glycogen synthase gene mutations. UK Prospective Diabetes Study. Diabetologia 39:1505–1511
Marcovina S, Bowsher RR, Miller WG et al (2007) Standardization of insulin immunoassays: report of the American Diabetes Association Workgroup. Clin Chem 53:711–716
Acknowledgements
The Botnia study was supported by a grant from the Sigrid Juselius Foundation. The EUGENE2 study was supported by the European Community (EUGENE2, n. LSHM-CT-2004-512013). We would like to thank A. Mari (ISIB-CNR, Padova, Italy), G. Mingrone (Università Cattolica Sacro Cuore, Rome, Italy), S. Salinari (University of Rome La Sapienza, Rome, Italy) and A. Kautzky-Willer (Medical University of Vienna, Vienna, Austria) for their help and suggestions. Preliminary results were presented at the EASD 2008 Annual Meeting in Rome, Italy.
Duality of interest
L. Groop has been a consultant for and served on advisory boards for sanofi-aventis, GSK, Novartis, Merck, Tethys Bioscience and Xoma, and received lecture fees from Lilly and Novartis. G. Pacini is currently consultant for Novo-Nordisk. All other authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00125-010-1740-x
Rights and permissions
About this article
Cite this article
Tura, A., Sbrignadello, S., Succurro, E. et al. An empirical index of insulin sensitivity from short IVGTT: validation against the minimal model and glucose clamp indices in patients with different clinical characteristics. Diabetologia 53, 144–152 (2010). https://doi.org/10.1007/s00125-009-1547-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-009-1547-9