Abstract
Aims/hypothesis
Childhood diabetes is thought to usually result from autoimmune beta cell destruction (type 1A) with eventual total loss of beta cells. Analysis of C-peptide in children characterised at diabetes onset for autoantibodies shows heterogeneous preservation of insulin secretion in long-standing diabetes. The aim of this study was to characterise the pancreases of childhood-onset diabetes in order to define the pathological basis of this heterogeneity.
Methods
We evaluated 20 cadaveric organ donor pancreases of childhood-onset long-term patients for disease heterogeneity and obtained corresponding C-peptide measurements.
Results
Pancreases from the majority of cadaveric donors contained only insulin-deficient islets (14 of 20). The remaining six patients (30%) had numerous insulin-positive cells within at least some islets, with two different histological patterns. Pattern A (which we would associate with type 1A diabetes) had lobular retention of areas with ‘abnormal’ beta cells producing the apoptosis inhibitor survivin and HLA class I. In pattern B, 100% of all islets contained normal-appearing but quantitatively reduced beta cells without survivin or HLA class I.
Conclusions/interpretation
Our data demonstrate that C-peptide secretion in long-standing diabetic patients can be explained by two different patterns of beta cell survival, possibly reflecting different subsets of type 1 diabetes.
Similar content being viewed by others
Introduction
Childhood-onset diabetes can result from multiple disease processes, but for the majority of children, diabetes results from immune-mediated beta cell destruction (type 1A) [1, 2]. Type 1A diabetes is characterised by the presence of anti-islet autoantibodies at onset [3] and extensive beta cell destruction as seen in pancreases of patients who died at onset. Particularly striking is the presence of insulin-deficient islets (islets with no beta cells). Childhood-onset diabetes may also result from uncommon forms of monogenic diabetes [4] (estimated to represent less than 2% of cases) and less well characterised forms such as ketosis-prone/‘Flatbush diabetes’, type 1.5 diabetes and type 2 diabetes [5]. In addition, neonatal-onset diabetes often results from mutations of the genes associated with beta cell signalling and proinsulin [6]. The Barbara Davis Center for Childhood Diabetes cares for approximately 80% of children with new-onset diabetes in the US state of Colorado. We have previously reported that, associated with Hispanic and African-American ancestry, approximately 15% of children presenting with new-onset diabetes produce no islet autoantibodies (e.g. GAD65, insulin, IA-2 or zinc transporter 8 [ZnT8]) [7]. In a recent study we also analysed C-peptide levels in patients with known autoantibody status at time of diagnosis and found that preservation of C-peptide in long-standing diabetes is more often (but not exclusively) seen in patients who were autoantibody-negative at onset [8]. The pathological basis of such heterogeneity in the preservation of C-peptide secretion in the different forms of long-standing childhood diabetes is not well characterised. In particular, it is not known how often type 1A diabetic patients have complete pancreatic beta cell loss [9–11] and whether beta cell loss occurs at all in the other variants of childhood diabetes.
The apoptosis inhibitor, survivin, is present in beta cells of fetal human islets [12] and is upregulated in the beta cells of patients with chronic pancreatitis [13]. Jiang et al. [14] recently reported that survivin is also present in postnatal mouse beta cells and is necessary for the expansion of beta cell mass. We have detected survivin in beta cells of NOD mice with beta cell mass higher than normal age-matched control SCID-NOD, despite the presence of significant insulitis (R. Gianani, unpublished data).
Therefore we analysed survivin levels by immunohistochemistry and immunofluorescence in the pancreases of normal controls and diabetic patients. In particular, we aimed to determine whether, similarly to NOD mice, survivin-positive beta cells accompanied by mononuclear islet infiltration could be detected. To begin addressing these questions, we analysed the pathology of pancreases (collected by the Juvenile Diabetes Research Foundation (JDRF)-sponsored nPOD initiative) from organ donors with a clinical history of childhood diabetes. Major advantages of the nPOD programme are that (1) the whole pancreas with multiple pancreatic blocks is available for analysis; and (2) the pancreatic autolysis associated with autopsy specimens is not present.
Methods
Selection of cases
The pancreases of patients with long-standing diabetes and controls (n = 31, consisting of 20 patients with childhood-onset diabetes and 11 non-diabetic controls) were obtained from organ donors (n = 28, including the 11 normal controls), autopsy cases (n = 2) or a surgical specimen (n = 1). This study was approved by the University of Colorado Institutional Review Board. Patients are referred to as DM1 to DM20, controls as C1 to C11. In DM19, a portion of the native pancreas was obtained at surgery for kidney–pancreas transplantation. The pancreases from organ donors were obtained through the JDRF-sponsored nPOD program. The nPOD program was created in 2007 to facilitate study of the pancreas from individuals with pre-diabetes or diabetes mellitus. It seeks cadaveric donor pancreases available for research from patients with type 1 diabetes (of any duration), those who are non-diabetic but are positive for anti-islet autoantibodies at the time of their death, and from non-diabetic controls. The mean age in our childhood-onset diabetes group was 25.6 years (range 4–49 years), the mean age of onset was 11.2 years (range 3–18 years) and the mean duration of diabetes was 14 years (range 1–35 years). This group consisted of 20 individuals (13 males, 7 females). Of the 20 childhood-onset patients, 13 were Non-Hispanic whites, one was multiracial, five were African-American and one was Hispanic. The control non-diabetic cadaveric donors consisted of 11 individuals with a mean age of 34.4 years (range 2–65 years). This group comprised six women and five men (nine whites, one African-American and one person for whom data on ethnicity could not be obtained).
An additional group of four pancreases from four male patients with a clinical diagnosis of type 2 diabetes (autopsy cases) was obtained from the archives of the University of Colorado Health Science Center Pathology Department. The mean age of this group was 51.2 years (range 22–64 years) at time of death.
Preparation of haematoxylin and eosin sections and immunostainining
For each childhood-onset case, the pancreas was processed in three regions (head, body and tail) and fixed overnight in 10% (vol./vol.) neutral buffered formalin (ThermoFisher, Waltham, MA, USA) prior to processing to paraffin blocks. Tissue sections (5 μm) were obtained stained with haematoxylin and eosin, immunohistochemistry and immunofluorescence. Pancreatic tissue sections were stained: (1) with antibodies to insulin or glucagon, and a panleucocyte marker CD45 and/or a T cell marker (CD3; and in a subset of diabetic pancreases with CD4, CD8, CD20 and CD68 antibodies); and (2) with a cocktail of antibodies to endocrine non-beta cell hormones composed of glucagon, pancreatic polypeptide and somatostatin. In diabetic pancreases with insulitis (as defined below), the infiltrating T cells were further characterised with antibodies to CD4 and CD8 (as well antibodies to CD20 and CD68 to detect B lymphocytes and macrophages).
Antibody binding was detected with appropriate secondary antibodies to guinea pig, rabbit and mouse immunoglobulins conjugated with alkaline phosphatase and peroxidase (MACH2 Polymer Systems; Biocare, Concord, CA, USA), followed by chromagen development with fast red (Vector, Burlingame, CA, USA) or diaminobenzidine (Vector). A scanner (CS ScanScope; Aperio, Vista, CA, USA) was used to produce whole slide images for both haematoxylin and eosin and immunohistochemistry-stained slides. In a subset of diabetic pancreases (pancreases containing residual beta cells), the pancreas was also double-stained with antibodies to insulin and class I HLA molecules.
Immunofluorescence was performed by incubating the tissue sections with antibody to insulin, glucagon and survivin (Abcam, Cambridge, MA, USA), and with appropriate anti-rabbit and anti-guinea pig immunoglobulins conjugated with aminomethylcoumarin acetate, Cy3 or Cy5. The sections were photographed at ×20 magnification using a microscope B651 (Olympus America, Center Valley, PA, USA) connected with a digital imaging system (Image pro plus, version 6.2; Media Cybernetics, Bethesda, MD, USA) with a camera (Pro 150ES; Pixera, San Jose, CA, USA). Additional images were recorded on an epifluorescence microscope (Microphot FXA; Nikon Instruments, Melville, NY, USA) with a monochrome digital camera (Roper Micromax; PerkinElmer, Waltham, MA, USA) and appropriate software (Intelligent Imaging Innovations, Denver, CO, USA). The photos are displayed in pseudocolour. Immunoperoxidase staining of survivin was performed by incubating the sections with antibody to survivin and visualising with Cytomation Envision+System-HRP (DAB; Dako, Carpenteria, CA, USA).
Class I HLA immunostaining was performed with mouse monoclonal anti-human HLA-ABC, Clone W6/32 (Dako), directed against a monomorphic epitope on the 45 kDa polypeptide products of the HLA-A, -B and -C loci. Insulin was detected with polyclonal guinea pig anti-swine insulin (Dako). All secondary goat antibodies (Life Technologies, Carlsbad, CA, USA) were highly cross-adsorbed against the host species of the other primary antibodies and conjugated with Alexa 488 (green) or 594 (red). Anti-fade reagent (Prolong Gold) with DAPI (Life Technologies) was applied upon staining and sections were imaged using an epifluorescence microscope (Eclipse 80i; Nikon) equipped with a mercury arc lamp (X-Cite, Mississauga, ON, Canada) and a digital camera (DXM1200C; Nikon). An air objective (×20; Nikon) was used with a 0.75 N.A.
Positive and negative controls for HLA-ABC staining included human spleen and isotope-matched primaries, respectively. Cross-reactivity with the insulin primary was also experimentally excluded.
In additional experiments, HLA immunofluorescence staining was performed with rabbit polyclonal antibody (Abcam). An irrelevant rabbit polyclonal antibody served as a negative control.
Morphometric analysis
Beta cell area (defined as the area of cells stained by insulin) was quantified using computer-assisted morphometric analysis of slides digitally stored in the Aperio System. We obtained the ratio between the insulin stained areas and the total pancreatic section area, i.e. the relative volume of beta cells in patients with diabetes and in normal controls. For each diabetic pancreas we then determined the beta cell index defined as the relative volume divided by the mean relative volume of beta cells of controls multiplied by 100. The beta cell index thus expressed the beta cell area of each diabetic pancreas as a percentage of normal control pancreases. Double immunofluorescent staining for insulin and glucagon was used to determine the percentage of islets devoid of beta cells in pancreases with residual beta cells (i.e. the percentage of insulin-deficient islets). In each section we determined the percentage of insulin-deficient islets (i.e. islets composed of non-beta cell vs the total number of islets). Only islets completely devoid of beta cells were considered insulin-deficient islets.
Assessment of insulitis
Insulitis was assessed by examining islets double-stained with antibodies to CD3 and/or CD45, and insulin. Only islets containing more than five mononuclear cells were considered positive for insulitis. Diabetic pancreases positive for insulitis were further characterised with antibodies to CD4, CD8, CD20 and CD68.
Islet autoantibody measurements
Serum specimens at the time of death were available for 19 of 20 childhood-onset type 1 diabetes patients. Islet cell autoantibodies to ICA512, GAD65 and ZnT8 were measured as previously described [7, 15]. Briefly, in vitro-translated antigens labelled with radioactive isotopes were immunoprecipitated with patient’s serum with positivity for autoantibodies set at a cut-off of the 99th percentile of normal controls.
Measurement of C-peptide levels
C-peptide measurements were performed by Northwestern Lipid Laboratories as previously described [16] for sera from cadaveric diabetic donors and normal controls. For one donor (DM19) C-peptide levels were obtained from clinical records. New-onset patient C-peptide levels were measured with a C-peptide assay (Alpco, Salem, NH, USA).
HLA typing
HLA-serological DR typing was obtained from the records of the organ donor organisations whenever possible. In some cases, HLA DR typing was obtained with high-resolution molecular typing.
Statistical analysis
Prisma software was used for statistical analysis with two sided contingency on t test.
Results
To better understand the pathology associated with retention of C-peptide, we analysed islet autoantibodies, C-peptide and pancreatic histology of patients who died with long-term diabetes. Table 1 summarises demographic data, as well as C-peptide, islet antibody status and HLA DR for each of the childhood-onset patients. As expected, C-peptide was not present in patients lacking histological evidence of beta cells (13 of 13). Of the six patients with extant beta cells, three had insulin-deficient islets. Of these three, one had no C-peptide (DM14) and the other two (DM13, DM15) had low C-peptide (both 0.06 nmol/l) (DM13 and DM15 were positive for anti-islet autoantibody at time of death). Of the other three patients with residual insulin-positive cells, none had insulin-deficient islets. Of the two in whom C-peptide could be measured at time of cadaveric donation, both had relatively high levels (DM18 and DM20, 7.32 and 0.67 nmol/l; respectively). The C-peptide mean value among normal controls (available for six normal controls) was 1.76 nmol/l; (range 0.93–10.49 nmol/l). For a summary of demographic data, C-peptide levels and islet antibody status for the normal controls, see Electronic supplementary material (ESM) Table 1.
In 70% (14 of 20) of pancreases from childhood-onset diabetic patients with diabetes duration of greater than 5 years, no insulin-positive cells were found within their islets. In this group, relative beta cell volume (area) was less than 1% of the mean of seven normal controls, with only rare single insulin-staining cells evident in pancreatic ducts or acinar regions. To account for any severely degranulated beta cells, beta cell area was confirmed by the lack of unstained islet cells after use of a cocktail of antibodies against glucagon, somatostatin and pancreatic polypeptide. In pancreases of these individuals, all islets were insulin-deficient (defined as islets without any beta cells). However, in pancreases of six patients, conserved beta cells were found in islets after 1, 8, 8, 11, 17 and 20 years of known diabetes respectively.
For pancreases with retained beta cells there were two fundamentally different patterns of histopathology (Table 1). The first, pattern A, was characterised by the presence of insulin-deficient islets and of islets retaining beta cells that contained the molecule survivin and had enhanced HLA class I abundance. Among patients with insulin-deficient islets (all but three having no conserved beta cells in the section), 13 of 17 were white, three were African-American (one was multiracial), and five of 17 were positive for islet antibodies at the time the pancreas was obtained (note that GAD65 and ICA512 islet autoantibodies are normally lost over time in patients with type 1A diabetes). In three patients (DM13, DM14, DM15), both insulin-deficient and insulin-positive islets were observed. Similar to descriptions by Gepts [11] of new-onset childhood patients who died at onset, insulin-positive islets had a ‘lobular’ pattern of distribution, being present only in some regions of the pancreas, with normal islets next to regions with insulin-deficient islets. The insulin deficient islets stained with an anti-glucagon antibody, but not with anti-insulin antibody (Fig. 1). Consistent with previous reports in new-onset patients, beta cells in all of the three patients with pattern A and residual beta cells showed increased levels of class I HLA molecules as determined with two separate antibodies (mouse monoclonal and rabbit polyclonal). HLA class I hyper production in alpha cells was present only in a subset of the insulin-deficient islets. However, all the islets containing insulin-positive cells hyper produced HLA Class I molecules (Fig. 2).
Immunostaining of a low-power section of the pancreas of cadaveric donor DM13 with antibody to glucagon (red staining) (a) and antibodies to insulin (also red staining) (b). Only two small areas in the section (rectangles) (b) contain insulin-positive islets; in contrast glucagon-positive islets are seen throughout the section
Immunofluorescence for insulin (a) and HLA class I ABC molecules (b) in the pancreas of DM14 (a patient with islet antibody positivity, insulin-deficient islets and residual beta cells). c Overlap of the two immunofluorescence stainings (a, b). HLA class I positivity is present in both the beta and non-beta cells of the pancreas
Consistent with our previous results [12], survivin was present in the alpha, but not in beta cells of normal adult human pancreas. Survivin was not detected in the pancreases of type 2 diabetic patients and in the pancreases of childhood-onset diabetic patients lacking insulin-deficient islets (DM18, DM19 and DM20); however, it was present in all beta cells within islets of all three pattern A pancreases (DM13, DM14 and DM15; Fig. 3). Triple immunofluorescence with insulin, glucagon and survivin confirmed the presence of survivin in beta cells of patients with pattern A beta cell loss, but not in patients with pattern B beta cell loss or in normal controls (Fig. 4).
Immunoperoxidase staining for survivin in the pancreas of a patient with childhood-onset diabetes and pattern A beta cell loss (DM13) (a), and in that of a normal control (C11) (b). Few cells (probably alpha cells, see below) are weakly stained in the pancreas of C11, whereas all endocrine cells in these insulin-containing islets from patient DM13 are stained
Immunofluorescence staining for survivin, insulin and glucagon in the pancreas of patients with pattern A beta cell loss (patient DM13) (a–f), pattern B beta cell loss (patient DM18) (g–l) and in a normal control (C10) (m–r). a, g, m Pseudocolour grey indicates insulin, glucagon and survivin respectively. d, j, p Insulin (pseudocoloured grey), survivin (pseudocoloured green) and glucagon-producing cells. e, k, q Glucagon (pseudocoloured grey), survivin (pseudocoloured green). f, l, r Merged composite images (insulin pseudocoloured blue, glucagon pseudocoloured red, survivin pseudocoloured green). Scale bars 50 µm. a–f Survivin staining is present in a subset of beta cells here, but not in other pancreases (g–l, m–r). The smaller percentage of stained beta cells seen in comparison to Fig. 5 probably reflects lower sensitivity of the immunofluorescence staining and differential producing of survivin by individual beta cells. In the pancreas of patient DM13 (a–f), survivin (a) is produced by beta and alpha cells, while in pancreases of patient DM18 (h) and C10 (o) beta cells do not produce survivin
The second pattern, pattern B, comprised the remaining three patients with islet beta cells (DM18, DM19 and DM20) in whom we did not detect any insulin-deficient islets and in whom 100% of islets had insulin-positive cells. Two of these patients were African-American and one was Hispanic. Two were also antibody-negative and had high C-peptide (0.67 and 7.32 nmol/l; missing in third patient due to lack of available sera) in contrast to the two (of 15) patients with insulin-deficient islets (p = 0.04). None of these three pattern B patients had high-risk HLA DR3 and/or DR4 alleles (vs 11 of 16, p = 0.05).
Insulitis was present in two of 20 of the patients and, as expected, both with retained beta cells and classed as pattern A. The pancreas containing islets affected by insulitis were obtained from patients DM14 and DM15. Thus we found insulitis in two (of three) patients with pattern A and residual beta cells. In contrast, we did not identify insulitis in any of the patients with pattern A histology and no residual beta cells, or in patients with pattern B histology. In both affected patients, cells manifesting insulitis were extremely rare, being identified in only four islets from DM15 and one islet from DM14 of the approximately 300 islets analysed for each case. In both cases the insulitis consisted prevalently of CD8+ cells (and in case DM15 a smaller number of CD20 cells and rare CD4+ lymphocytes and macrophages). There was no difference in the relative beta cell volume in pancreases with and without insulitis (among the six pancreases with conserved beta cells; p = 0.79).
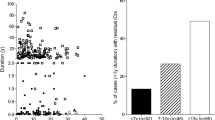
The pattern B pancreatic histology observed in patients DM18, DM19 and DM20 was similar to that of older patients with reported type 2 diabetes (n = 4) in terms of lack of insulin-deficient islets and lack of survivin beta cell production (see below). Figure 5 shows the pattern of beta cell distribution in the pancreas of patient DM20, which, like that of other patients with pattern B, was characterised by a diffuse decrease of beta cells and lack of insulin-deficient islets. However, the beta cell relative volume in patients with pattern B childhood-onset diabetes was reduced in comparison both with the type 2 diabetes patients analysed and with normal controls (p = 0.02 vs type 2 diabetes, p = 0.004 vs controls; Fig. 6). Figure 7 plots the percentage of insulin-positive islets for the pancreases analysed.
a Immunoperoxidase staining with insulin of a pancreatic islet of patient DM20 (who had dramatic pattern B beta cell loss); only a small subset of islet cells are stained for insulin (brown). b A different islet stained by immunofluorescence with antibodies to insulin (blue) and glucagon (red). The majority of the endocrine cells in this islet are stained for glucagon. A similar pattern was observed in all the pancreatic islet of this patient
Beta cell islet area (expressed as percentage of mean beta cell islet area in normal controls) in the pancreases of: controls (black diamonds); long-standing childhood-onset diabetic patients with pattern A (black squares) and pattern B (black triangles); and patients with type 2 diabetes (grey diamonds) and normal controls (grey triangles). The majority of the childhood-onset pancreases did not contain any insulin-positive islets, but six patients had residual insulin-positive islets
Percentage of islets containing insulin-positive cells in the pancreases of: normal controls (black squares); patients with childhood-onset diabetes pattern A (black triangles) and pattern B (white squares); and patients with adult-onset type 2 diabetes (white triangles) and normal controls (black circles). Only three (of six) pancreases of patients with childhood-onset diabetes had residual beta cell area as well as insulin-deficient islets (i.e. islets without any insulin-positive cells) (pattern A). The remaining three pancreases of patients with childhood-onset diabetes with residual beta cells (as well as the pancreases of patients with a clinical history of type 2 diabetes) did not contain any insulin-deficient islets (pattern B). In the chart these individuals are shown as having 100% of their islets containing insulin-positive cells. The majority (15 of 18) of the pancreases of childhood-onset diabetic patients contained insulin-deficient islets, including the two patients who also had islets with insulin-positive cells. All these patients, except for the two with residual beta cells, are shown as having 0% of their islets containing insulin-positive cells
There was no difference in BMI between patients with pattern A and pattern B beta cell loss. Among patients with pattern B, DM18 had a high BMI (30.2); in comparison, pattern A patient DM13 had an even higher BMI (30.9). The average BMI of normal controls (available for ten of 11 controls) was 24.5 (range 15.7 to 32.2) kg/m2.
Review of the clinical information for patients with pattern B revealed that all three patients were thought to have type 1 diabetes clinically. Two of them had end-stage renal disease (DM18, DM20). In one patient (DM20), from whom the pancreatic sample was obtained during double kidney–pancreas transplantation, diabetes was very difficult to control with insulin treatment. Since pancreatic transplantation in 2006, this patient has remained euglycaemic with a fasting C-peptide of 1.02 nmol/l and glycosylated haemoglobin of 5.38% (laboratory data obtained October 2009). The third patient with pattern B was admitted to the hospital for diabetic ketoacidosis and subsequently died from cerebral oedema.
Discussion
In the current study we have demonstrated that in most patients with childhood-onset diabetes, the pancreas contains few, if any insulin-positive beta cells, with all islets being insulin-deficient and having a few scattered and isolated (mostly ductal) insulin-positive cells (all these patients were negative for C-peptide). Nevertheless, three (of 17; 17%) pancreases with regions of insulin-deficient islets also had islets with beta cells, in two of which (from patients with 20 years disease duration and onset at age 17, and 1 year disease duration with onset at age 12 respectively) a low level of C-peptide was detectable at time of organ donation. The age of onset, presence of high-risk HLA alleles, lack of C-peptide in serum at time pancreas was obtained and islet autoantibody positivity suggest that such patients have type 1A diabetes, defined as immune-mediated diabetes. Two of the white patients with retained islet beta cells, insulin-deficient islets and islet autoantibodies almost certainly had type 1A diabetes, given their islet autoantibody positivity. These patients had low C-peptide secretion (0.06 nmol/l) and undetectable C-peptide (DM15 and DM14 respectively). The other white patient with retained beta cells and insulin-deficient islets also had low C-peptide (0.06 nmol/l). The beta cells of all patients with pattern A histology were abnormal. All beta cells of patients with pattern A produced survivin and showed enhanced HLA-class I by immunohistochemistry. A similar pattern of beta cell loss, characterised by the lobular distribution of insulin-deficient islets, was seen in these two patients with retained beta cells. This lobular pattern of beta cell loss has been described in the pancreas of patients who died with new-onset type 1A diabetes [9]. In the pancreatic zones containing only insulin-deficient islets, the acinar pancreas also appeared atrophic, consistent with the well documented atrophy of the pancreas in type 1 patients [10]. Despite the presence of beta cells, we found lymphocytic infiltrate in only two of three patients with pattern A after analysing multiple sections (25 sections for one, three sections for the other patient) from different areas of the pancreas. Consistent with previous reports, insulitis was composed mostly by CD8-positive lymphocytes [17].
In the two African-American and one Hispanic cadaveric donor with long-standing childhood-onset diabetes, histopathology was readily distinguishable from that of donors with pattern A in that they retained beta cells in 100% of islets examined, had normal or even high levels of C-peptide, and lacked both HLA class I hyper-production in beta cells and survivin. The pancreatic pathology in these patients was similar to that of adult-onset type 2 diabetes, where the pancreas also lacks insulin-deficient islets, but these patients had lower beta cell area. A larger number of pancreases of childhood-onset and type 2 diabetic patients will need to be analysed to determine whether there is a consistent decrease in beta cell area in these patients compared with patients identified as having adult-onset type 2 diabetes [18].
The apoptosis inhibitor survivin is produced in beta cells of fetal human pancreas [12], but not in adult islets. It is also found in the beta cells in areas of pancreatitis [13]. The production of survivin, which was present within lobular areas in all surviving islet beta cells of all islets of pancreas of presumed type 1A (immune-mediated) patients, might result from intermittent inflammation that did not result in beta cell destruction of a subset of islets. Alternatively beta cells that produce survivin might be protected from destruction and further lymphocytic infiltration. An alternative hypothesis is that lobular regions with beta cells of pattern A pancreas represent regeneration.
In summary we believe that the presence of insulin-deficient islets, survivin and class I HLA in beta cells (pattern A) in long-term childhood-onset diabetes is a common hallmark of type 1A diabetes in the subset of patients with residual beta cells. In contrast, lack of insulin-deficient islets, with variable beta cell loss within individual islets (but with all islets having some beta cells), marks other forms of childhood-onset diabetes, including type 2 diabetes; in the current series, this second pattern was present in three of 20 childhood-onset patients. The drive to understand (1) mechanisms leading to retention of beta cells with survivin production in patients with long-term type 1A diabetes and (2) the spectrum of causes of childhood-onset diabetes with pattern B should benefit from programmes such as nPOD. To foster further studies of this unique resource, the nPOD histology will be available online (www.jdrfnpod.org; with availability of tissue) to interested investigators.
Abbreviations
- JDRF:
-
Juvenile Diabetes Research Foundation
- ZnT8:
-
Zinc transporter 8
References
Spencer J, Peakman M (2009) Post-mortem analysis of islet pathology in type 1 diabetes illuminates the life and death of the beta cell. Clin Exp Immunol 155:125–127
Dotta F, Eisenbarth GS (1989) Type I diabetes mellitus: a predictable autoimmune disease with interindividual variation in the rate of beta cell destruction. Clin Immunol Immunopathol 50:85–95
Gianani R, Eisenbarth GS (2005) The stages of type 1A diabetes: 2005. Immunol Rev 204:232–249
Vaxillaire M, Bonnefond A, Froquel P (2009) Breakthroughs in monogenic diabetes genetics: from pediatric forms to young adulthood diabetes. Pediatr Endocrinol Rev 6:405–417
Maldonado M, Hampe CS, Gaur LK et al (2003) Ketosis-prone diabetes: dissection of a heterogeneous syndrome using an immunogenetic and beta-cell functional classsification, prospective analysis, and clinical outcomes. J Clin Endocrinol Metab 88:5090–5098
Njolstad PR, Sovk O, Cuesta-Munoz A et al (2008) Neonatal diabetes mellitus due to complete glucokinase deficiency. N Engl J Med 344:1588–1592
Wang J, Miao D, Babu S et al (2007) Prevalence of autoantibody-negative diabetes is not rare at all ages and increases with older age and obesity. J Clin Endocrinol Metab 92:88–92
Steck KA, Yu L, Armstrong T et al (2009) C-peptide levels correlate with age of onset, duration of diabetes and autoantibody status. Diabetes 58(Suppl 1):A459 (Abstract)
Foulis AK, Liddle CN, Farquharson JA, Richmond JA, Weir RS (1986) The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia 29:267–274
Lohr M, Kloppel G (2004) Residual insulin positivity and pancreatic atrophy in relation to duration of chronic type 1 (insulin-dependent) diabetes mellitus and microangiopathy. Diabetologia 30:757–762
Gepts W (1965) Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14:619–633
Liggins C, Orlicky D, Bloomquist L, Gianani R (2008) Developmentally regulated expression of survivin in human pancreatic islets. Pediatr Dev Pathol 6:392–397
Hasel C, Bhanot UK, Maier R, Strater J, Moller P (2005) Parenchymal regression in chronic pancreatitis spares islets reprogrammed for the expression of NF kappa B and IAPs. Lab Invest 85:1263–1275
Jiang Y, Nishimura W, Devor-Henneman D et al (2008) Postnatal expansion of the pancreatic beta-cell mass is dependent on survivin. Diabetes 57:2718–2727
Wenzlau JM, Juhl K, Yu L et al (2007) The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabets. PNAS 104:17040–17045
Chen J, Munter P, Hamm LL et al (2003) Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol 4:469–477
Foulis AK (2008) Pancreatic pathology in type 1 diabetes in humans. Novartis Found Symp 292:2–13
Yoon KH, Ko SH, Cho JH et al (2003) Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 88:2300–2308
Acknowledgements
We gratefully acknowledge the JDRF-sponsored nPOD programme and the Brehm Coalition, which supported this study. Some of the experiments were performed by the Diabetes Endocrinology Research Center histology core (P30DK057516), NIH DK 32083. S. A. Sarkar is supported by NIH DK080193 and JDRF 1-2008-1021.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00125-010-1769-x
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM Table 1
Demographic information and laboratory values as shown in the 11 non-diabetic controls analysed in this study (PDF 14 kb)
Rights and permissions
About this article
Cite this article
Gianani, R., Campbell-Thompson, M., Sarkar, S.A. et al. Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia 53, 690–698 (2010). https://doi.org/10.1007/s00125-009-1642-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-009-1642-y