Abstract
Aims/hypothesis
Immunohistochemical staining reveals that the enteroviral capsid protein VP1 is present at higher frequency in the insulin-containing islets of patients with recent-onset type 1 diabetes than in controls. This is consistent with epidemiological evidence suggesting that enteroviral infection may contribute to the autoimmune response in type 1 diabetes. However, immunostaining of VP1 is not definitive since the antibody widely used to detect the protein (Clone 5D8/1) might also cross-react with additional proteins under some conditions. Therefore, we sought to verify that VP1 immunopositivity correlates with additional markers of viral infection.
Methods
Antigen immunoreactivity was examined in formalin-fixed, paraffin-embedded, pancreases from two different collections of type 1 diabetes and control cases: a historical collection from the UK and the nPOD (network of Pancreatic Organ donors with Diabetes) cohort from the USA.
Results
VP1 immunoreactivity was present in ∼20% of insulin-containing islets of both cohorts under stringent conditions but was absent from insulin-deficient islets. The presence of VP1 was restricted to beta cells but only a minority of these contained the antigen. The innate viral sensor, protein kinase R (PKR) was upregulated selectively in beta cells that were immunopositive for VP1. The anti-apoptotic protein myeloid cell leukaemia sequence-1 (Mcl-1) was abundant in beta cells that were immunonegative for VP1 but Mcl-1 was depleted in cells containing VP1.
Conclusions/interpretation
The presence of immunoreactive VP1 within beta cells in type 1 diabetes is associated with a cellular phenotype consistent with the activation of antiviral response pathways and enhanced sensitivity to apoptosis. However, definitive studies confirming whether viral infections are causal to beta cell loss in human diabetes are still awaited.
Similar content being viewed by others
Introduction
Considerable circumstantial evidence has accumulated to suggest that enteroviral infection of pancreatic islet beta cells may contribute to the development of autoimmunity in some patients with type 1 diabetes [1–4]. This is important since, if verified in a majority of cases, it may provide a means to minimise the future development of type 1 diabetes in susceptible individuals via a targeted vaccination programme.
The evidence implicating enteroviral infection of pancreatic beta cells has arisen from a variety of sources including the successful culture of live enteroviral strains from within the pancreases of individuals with type 1 diabetes and the capture of electron microscopic images showing the generation of viral arrays within the beta cells of patients [5, 6]. Additionally, a common enteroviral capsid protein, VP1, has been detected immunologically in the islet cells of patients with recent-onset type 1 diabetes, at much higher frequency than in age-matched controls [5, 7]. Arguably, this latter evidence is the more persuasive for several reasons. In particular, it has been noted that the enteroviral strains isolated during pancreas culture are of uncertain provenance [8] and the electron microscopic evidence of viral-particle assembly is derived from only a very small number of cases [5]. By contrast, enteroviral VP1 was detected at high frequency in the islets of more than 60% of patients with recent-onset type 1 diabetes in a large UK cohort [7]. Importantly, however, it must also be recognised that the immunolabelling of enteroviral VP1 in islet cells remains equivocal since the specificity of the principal antibody employed in these studies (Dako 5D8/1) has been questioned (although its ability to detect enteroviral VP1 is not the subject of debate) [9]. Furthermore, the presence of viral antigen is detected much more readily than viral RNA in islet cells under most conditions. Therefore, it is important that additional evidence is gained to evaluate further the hypothesis that the immunodetection of enteroviral VP1 in the islet beta cells of patients with recent-onset type 1 diabetes is associated with an underlying viral infection.
In this study we addressed this issue in two ways. First, we made use of a second (much more modern) collection of pancreas samples from within the JDRF nPOD (Juvenile Diabetes Research Foundation network of Pancreatic Organ donors with Diabetes) programme [10] and compared the staining patterns obtained using the 5D8/1 anti-VP1 serum in these samples with that obtained previously in the historical UK cohort. This is important since the UK samples were collected 30–50 years ago and were harvested at autopsy in random locations across the UK, where entirely different methods of recovery and fixation were employed [11]. By contrast, the nPOD programme was established much more recently (the collection began in 2007) in a geographically distinct region (the USA) and uses standard operating procedures to ensure consistency of recovery and processing of the samples [10]. Second, we evaluated whether, in either of the cohorts, the detection of VP1 at the level of individual islet cells under stringent conditions, is directly correlated with additional markers consistent with the establishment of an antiviral response. In particular, we monitored the expression of two further proteins, protein kinase R (PKR) and myeloid cell leukaemia sequence-1 (Mcl-1), in concert with VP1. PKR is an enzyme responsible for the activation of antiviral cascades within cells and is induced during enteroviral infection of cells [12, 13]. Mcl-1 is an anti-apoptotic protein that is subject to rapid turnover in cells, such that it is degraded quickly during the translational arrest that ensues following viral infection [14]. Accordingly, we present a detailed analysis of the correlations between the immunodetection of VP1, induction of PKR and the level of Mcl-1 in individual beta cells in patients with type 1 diabetes.
Methods
Subjects
Pancreases recovered from six patients with recent-onset type 1 diabetes mellitus (disease duration ≤18 months) and one with type 1 diabetes of 12 years’ duration were selected from within a UK cohort used previously [7]. The specimens were studied with ethics approval (obtained as part of the previous study [7]) and had been variously fixed in buffered p-formaldehyde, unbuffered formol saline or Bouin’s fixative. They were all paraffin-embedded. Five of the seven pancreases were removed at autopsy and two were recovered at the time of organ donation (D4 and D5). The patients were typically between 1 and 18 years old but one was aged 42 years, raising the overall mean (±SD) age to 19.0 ± 5.0 years (electronic supplementary material [ESM] Table 1). A second cohort of pancreases from 17 patients with type 1 diabetes mellitus (mean age 25.7 ± 2.9 years) and 12 age-matched non-diabetic controls (mean age 27.9 ± 7.1 years) was obtained through the JDRF nPOD programme (Table 1, ESM Table 2) [10]. Ethics approval for the nPOD cohort was obtained by Campbell-Thompson et al [10].
Immunohistochemistry
Sections (4 μm; mounted on glass slides coated in [3-aminopropyl]-triethoxysilane; Sigma, Poole, UK) were processed and labelled using a standard immunoperoxidase technique [7, 15, 16]. With the exception of insulin, all antigens were unmasked by heating in 1 mmol/l EDTA (Sigma) buffer pH 8.0, in a pressure cooker in a microwave oven on full power (800 W) for 20 min, followed by 20 min of cooling at room temperature. Primary antibodies were applied, as detailed in ESM Table 3, and Dako REAL Envision Detection System (Dako, Ely, UK) was used for antigen detection. Some slides were processed in the absence of primary antibody or with isotype control antisera to confirm the specificity of labelling. Slides were analysed by light microscopy.
Combined immunofluorescence
For co-localisation studies, anti-insulin and anti-VP1 immunoreactivity were detected using an AlexaFluor 488-conjugated anti-guinea pig antibody and an AlexaFluor 568-conjugated anti-mouse antibody, respectively (Invitrogen, Paisley, UK). To determine whether the enteroviral VP1 protein co-localised with either PKR or Mcl-1 in beta cells, primary antibodies were incubated as described in ESM Table 3. The primary antibodies were detected with relevant goat secondary antibodies conjugated to AlexaFluor 488 or 568 (Invitrogen) or with goat anti-guinea pig DyLight 405 (Stratech, Newmarket, UK). Control sections were stained with relevant primary and secondary antisera to confirm that no cross-reactivity was detected. Sections were mounted in Vectashield hard-set mounting medium (Vector Laboratories, Peterborough, UK) under glass coverslips. Images were captured using a Nikon Eclipse 80i microscope (Nikon, Kingston upon Thames, UK) and overlaid using NIS-Elements BR 3.0 software (Nikon) to study the relative localisation of each antigen. Sections directly adjacent to those stained using the combined method were stained with an anti-glucagon (rabbit; Dako) or an anti-insulin (guinea pig; Dako) antibody using a standard immunoperoxidase technique to determine total islet numbers in the sections and to distinguish insulin-containing islets (ICIs) from insulin-deficient islets (IDIs).
Results
Enteroviral VP1 is frequently detected in islets of type 1 diabetes patients from both the UK and nPOD cohorts
Previous analysis of the UK cohort had revealed the presence of the enteroviral VP1 protein in the ICIs of 44 of 72 (61%) recent-onset type 1 diabetes patients. This compared with only 3 of 50 paediatric and neonatal controls [7]. The pancreas samples collected from patients with recent-onset type 1 diabetes within the UK cohort are unique in that they represent about half of all such cases described worldwide and because they come from individuals with short duration of disease. However, they were harvested between 30 and 50 years ago and were fixed using non-standardised protocols [11]. We considered it important, therefore, to establish whether enteroviral VP1 staining was also present in a different cohort, collected more recently, from a geographically different population, using more standardised protocols. The JDRF nPOD collection provided this opportunity [10]. A comparison of the samples available within these two cohorts is shown in Table 2 and it is noteworthy that the donor with the shortest duration of disease among the nPOD samples had been diabetic for 1 year. Moreover, the mean disease duration among this group was approximately 12 years. This compares with a mean disease duration of less than 1 year in the UK cohort (Table 2).
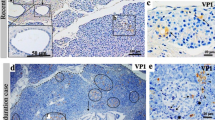
Initial experiments using an enteroviral VP1 antibody (5D8/1) were performed to determine the optimal concentration for routine use that allowed clear immunodetection of VP1 with minimal background staining. Seventeen type 1 diabetes nPOD cases and 12 non-diabetic controls were then immunostained (Table 1, ESM Table 2). Among the nPOD controls, only one case was found in which a combined total of more than five individual islet cells were immunopositive for VP1 in the entire pancreas section. Accordingly, this value was chosen to represent the threshold limit to define immunopositivity for VP1 in control samples. By definition, then, one of 12 (8.3%) non-diabetic controls was judged to be immunopositive for VP1 using this criterion (Fig. 1). By contrast, multiple intensely VP1-positive islet cells were observed in many ICIs in eight of the 17 nPOD type 1 diabetes cases. Of the remaining nine cases, seven were immunonegative for insulin. Hence, VP1 was detected at well above the threshold level in the islet cells of eight of the ten nPOD cases (80%) in which ICIs were present (Fig. 1, Table 1). Representative VP1-positive ICIs for both cohorts are shown in Fig. 1a, b and the data are presented graphically in Fig. 1c.
Representative immunohistochemical images of enteroviral VP1 levels in the UK (donor D4) (a) and nPOD (donor 6052-01) cohorts (b). (c) The proportion of cases with islets staining intensely positive for VP1. White bars, non-diabetic controls (UK n = 50 [7]; nPOD n = 12); hatched bars, type 1 diabetes cases with ICIs (UK n = 72 [7], nPOD n = 10); black bars, type 1 diabetes cases containing only IDIs (nPOD n = 7)
The absolute number of enteroviral VP1-positive islet cells is elevated in organ donor cases by comparison with those recovered at autopsy
Quantification of the frequency of VP1-positive ICIs in seven VP1-positive nPOD cases and seven VP1-positive UK cases was performed by counting the total number of islets containing one or more VP1-positive cells within a given section. Analysis of both cohorts revealed that 65 of 227 (28.6%) islets from within the nPOD cases contained VP1-positive cells; this compared with 77 of 374 (20.6%) islets within the UK cohort (Fig. 2a, Table 1, ESM Table 1). Therefore the frequency of VP1-immunopositive ICIs was similar in the two cohorts. However, when the absolute number of VP1-positive cells within any given islet was studied and compared between the two cohorts, initial evidence implied that this number might be elevated in the nPOD cases (Fig. 1). Therefore, to assess this more formally, a minimum of 20 randomly selected VP1-positive islets were imaged from among the nPOD organ donors (nPOD OD), UK organ donors (UK OD) and UK post-mortem (UK PM) cases. The total number of endocrine cells contained within the imaged islets was recorded and correlated with the number of VP1-positive endocrine cells. This revealed a significant increase in the percentage of individual VP1-positive endocrine cells present within any given islet among the organ donor samples (nPOD OD, 5.52 ± 0.90%; UK OD, 5.10 ± 0.87%; Fig. 2b) compared with the post-mortem cases (1.76 ± 0.32%; p < 0.001). Therefore the frequency of VP1-positive islet cells seen in organ donors was similar in both cohorts (whether from the USA or the UK).
(a) Proportion of ICIs with VP1-positive cells in 166 ICIs from the nPOD cohort (black bars) and from 374 ICIs from the UK cohort (white bars). (b) Percentage of total endocrine cells that are VP1-positive in UK PM cases (white bars; n = 21 islets), UK OD cases (black bars; n = 20 islets) and nPOD OD (hatched bars; n = 25 islets). Data are presented as mean values ± SEM
Enteroviral VP1 co-localises with insulin in both the UK and the nPOD cohort
The observation that VP1-positive islet cells were confined only to those nPOD cases in which ICIs were also present, implies that VP1 might be preferentially localised within beta cells (as was reported previously for the UK cohort [7, 17]). To confirm this, three of the nPOD VP1-positive cases were stained for the presence of both VP1 and insulin using dual immunofluorescence. A total of 31 VP1-positive ICIs were identified. Overlaying the fluorescent images (Fig. 3) revealed that VP1 immunopositivity always co-localised with insulin. A similar situation existed within the UK cohort. Interestingly, it was noted anecdotally that the intensity of insulin staining appeared to be reduced in the VP1-positive beta cells when compared with surrounding VP1-negative beta cells.
Photomicrographs of representative islets from the nPOD (donor 6052-01; a, c, e) and UK (donor D4; b, d, f) cohorts reveals that VP1 (red; c, d) co-localises with insulin (green; a, b). Double-positive cells are stained yellow and are visible in (e) and (f). Nuclear DAPI staining is shown in blue in the merged images (e, f)
Enteroviral VP1 co-localises with PKR in patients with type 1 diabetes
Enhanced levels of the pathogen recognition receptor PKR have been observed in VP1-positive islets within the UK recent-onset type 1 diabetes patients [7] although it was not established whether VP1 and PKR are preferentially localised within the same cells. To study the relative localisation of VP1 and PKR, dual immunofluorescence staining was performed in pancreas samples from both the UK and the nPOD cohorts. Within the seven UK type 1 diabetes patients, 374 of 866 islets (43.1%) contained cells that were immunopositive for insulin, and VP1 was detected in 77 of these. Dual immunofluorescence staining confirmed that the VP1-positive cells always co-produced PKR. A representative image of one such islet is presented in Fig. 4. Similar co-localisation of VP1 and PKR within individual beta cells was also observed in the 31 VP1-positive islets of the nPOD type 1 diabetes cases (Fig. 4). PKR abundance in the surrounding cells tended to be slightly elevated with respect to the background in the nPOD cases when compared with the UK cohort, where it was only the VP1-positive cells that displayed clear immunoreactive PKR levels.
Photomicrographs of representative islets from the nPOD (donor 6084-01; a, c, e) and UK (donor D4; b, d, f) cohorts reveals that VP1 (green; a, b) co-localises with PKR (red; c, d). Double-positive cells are stained yellow and are visible in (e) and (f). Nuclear DAPI staining is shown in blue in the merged images (e, f)
Mcl-1 is present in beta cells but its production is reduced in those cells containing VP1
PKR exists in both active (phosphorylated) and inactive (dephosphorylated) forms but it may be misleading to infer changes in specific protein phosphorylation patterns by immunological means in formalin-fixed paraffin-embedded (FFPE) tissue due to potential changes arising during tissue fixation and processing. Therefore, we employed an alternative approach to assess the activity status of PKR in the islet cells of patients with type 1 diabetes. This was based on the understanding that the anti-apoptotic protein Mcl-1 is subject to rapid turnover in cells and that its levels decline quickly under conditions when protein translation is attenuated [18]. Since an important consequence of the activation of PKR is to inhibit protein translation in response to viral infection (via phosphorylation of eukaryotic translation initiation factor 2 alpha (eIF2α)) we reasoned that Mcl-1 levels should decline in cells where PKR becomes activated. Therefore, the levels of Mcl-1 were evaluated in human FFPE pancreatic tissue and, more specifically, we examined the intensity of immunostaining of Mcl-1 in VP1-positive cells (which, as shown above, always have elevated PKR) within the islets of the type 1 diabetes cases. Immunoperoxidase staining of non-diabetic control pancreas revealed intense staining of a subset of islet endocrine cells (Fig. 5), confirming the presence of Mcl-1 within certain islet cells. Dual immunofluorescence labelling revealed that these were beta cells (Fig. 5). Importantly, the intensity of staining of Mcl-1 was markedly reduced (and was frequently undetectable) in those islet cells that were immunopositive for VP1. Example images are shown in Fig. 6. To verify these data, 152 individual VP1-positive islet cells were examined across a total of eight different type 1 diabetes cases and scored for the presence of Mcl-1. Mcl-1 immunostaining was reduced (or was undetectable) in all cells containing VP1 whereas Mcl-1 was stained much more intensely in adjacent, VP1-immunonegative, beta cells.
(a) Light microscopic image of Mcl-1 levels in an islet from a non-diabetic control individual demonstrates intense staining of a subset of islet endocrine cells. (b–d) Fluorescence microscopic analysis of a representative islet from a UK case reveals that Mcl-1 (red; c) co-localises with insulin (green; b). Double-positive cells are stained yellow and are visible in (d). Nuclear DAPI staining is shown in blue in the merged images (d)
Photomicrographs of a representative islet from a UK case reveals that the VP1-positive cells (green staining outlined in yellow; a) have reduced levels of Mcl-1 (red; b) when compared with surrounding beta cells that are shown to contain high levels of Mcl-1 (purple in merged image; c). Autofluorescent erythrocytes are indicated by asterisks
Discussion
Previous studies have shown that, in patients with recent-onset type 1 diabetes, a proportion of islets contain cells in which the enteroviral capsid protein, VP1, can be detected by immunocytochemistry [5, 7]. This conclusion was first drawn by Dotta et al based on studies of five patients, with type 1 diabetes, from Italy [5] and was substantiated by the analysis of a much larger cohort of pancreatic tissue collected from within the UK. These had been harvested from patients (mainly children) who died between 30 and 50 years ago and among whom the mean duration of diabetes was 8 months [7]. In the present work, it is shown that pancreas samples that were collected from 2007 onwards from a third group of patients with type 1 diabetes (who died within the USA) also contain islets that stain positively for enteroviral VP1. A group of relevant age-matched controls was employed to verify the selectivity of immunolabelling in each cohort. The results revealed that while VP1 is occasionally detected in the islets of non-diabetic individuals, it is found much more abundantly in the cells of patients with type 1 diabetes. As such, these data support the earlier conclusion that enteroviral infection of islet cells may be a characteristic feature of patients with type 1 diabetes in the western world [7, 19]. The current study also reveals that this feature is seen not only in patients with recent-onset disease but also in a group with longer-standing type 1 diabetes, since the mean duration of diabetes since diagnosis in the nPOD samples analysed was almost 12 years. This, in turn, reinforces the view that the nature of the infection occurring in the islet cells in type 1 diabetes is unusual in that it may persist over long periods rather than developing as an acute, lytic infection which is more typical of enteroviruses [19]. The results also show that both the historical UK collection and the nPOD samples represent suitable material for analysis of this infection in detail.
The deduction that the enteroviral infection is persistent may offer an explanation for our observation that the enteroviral capsid protein, VP1, is found in relatively few of the total number of islet cells available within each patient. As such, it has been proposed that detection of VP1 may be simply the ‘tip of an iceberg’ in which many more cells harbour a latent infection and that, occasionally, this is manifest by the activation of viral protein synthesis within defined endocrine cells [19]. Mechanisms by which enteroviruses can persist in a latent form without causing large-scale cell lysis have been described [20–24] and we have confirmed in preliminary work that cultured mammalian cells that harbour such persistent infections in vitro are not routinely immunopositive for VP1 (S. J. Richardson, N. M. Chapman, unpublished observations). However, the mechanism by which viral persistence is achieved in type 1 diabetes has not been defined and must await the final isolation and characterisation of the relevant viral serotype(s).
A second important consideration arising from this work is that immunodetection of VP1 should be undertaken only after rigorous optimisation of the staining pattern in pancreas samples. The most widely employed and robust antiserum available currently for immunodetection of VP1 in FFPE tissue samples is the 5D8/1 monoclonal serum (Dako). However, we have noted that, in pancreas samples from non-diabetic and diabetic individuals from both the UK and nPOD cohorts, this serum can generate intense positivity in a wide range of endocrine and exocrine cells when employed at low dilution [7]. By contrast, when titrated to high dilution, the majority of this staining is lost and only occasional single, strongly immunopositive cells remain. In both cohorts these were exclusively beta cells, as judged by the pattern of co-staining with antisera to islet hormones. This is consistent with the earlier finding that beta cells express surface receptors capable of mediating enteroviral entry [25, 26].
It is of interest that the dilution of clone 5D8/1 required for optimal immunostaining of nPOD samples was much higher (1:2,000) than that which proved optimal for the UK cohort (1:500) and this may reflect the better tissue preservation of the former samples. In support of this, it was noted, too, that the mean number of VP1-immunopositive cells per islet (>5%) was greater in those samples recovered from organ donors (where tissue preservation was at a premium) than in those collected at autopsy (<2%; where tissue preservation is less controlled). This finding held true both among the older UK cohort and within the more-recent nPOD samples, such that the mean number of VP1-immunopositive cells per islet was similar in both organ donor groups (5.1% vs 5.5% respectively). This could be taken to imply that the frequency of enteroviral infection of beta cells does not change dramatically over time or with geographical location.
A further critical issue is the identity of the immunoreactive protein labelled by the anti-VP1 serum in the samples studied here. It is well-accepted that clone 5D8/1 binds to enteroviral VP1 encoded by a wide range of viral serotypes, but there remain hints in the literature that it may also bind to additional proteins (albeit with lower affinity) under certain conditions [9, 27]. Indeed, as indicated above, we found that, at low dilution, the serum stains multiple cells (within and outside islets) in both control and type 1 diabetes patients [7] but that this issue is resolved at higher dilution. Nevertheless, it cannot be formally excluded that an additional (non-viral) target is labelled even under the more stringent conditions. However, if this is the case, then we now show that this response only occurs in cells that display certain critical additional features. In particular, we have studied samples from both the UK and nPOD cohorts and find that, remarkably (and without exception), beta cells that are immunopositive for VP1 also show intense immunostaining of the pathogen recognition receptor, PKR. This implies that the total amount of PKR protein is enhanced in these cells, presumably via induction of mRNA synthesis and translation. Previously, we had provided evidence (using serial sections examined by immunohistochemistry) that the same islets are stained positively by both antisera [7] but the present work now reveals that this staining is co-localised within the same individual beta cells. Hence, it can be concluded that these specific cells are mounting a response to an insult that is directly associated with VP1 production. Since enteroviral infection has been shown to markedly upregulate PKR production in islet cells at the mRNA level [12, 13], the precise conjunction of the two phenomena monitored here adds weight to the conclusion that VP1 abundance is representative of underlying enteroviral infection. Having made this point, however, it must also be accepted that, in principle, the production of PKR might also be induced by other (non-viral) stressors in beta cells. However, if this is the case, then it still remains true that the anti-VP1 serum must be capable of selectively labelling a unique subset of beta cells which are experiencing atypical stress.
To advance these arguments further, we also investigated the status of Mcl-1 abundance in islet cells in type 1 diabetes. Mcl-1 is an anti-apoptotic member of the Bcl-2 protein family that, due to unique motifs in its N-terminal domain, is subject to rapid turnover under normal conditions [18]. Therefore, to maintain adequate levels of Mcl-1 within the cell, the protein must be synthesised and replenished at a rate that is at least equal to its rate of degradation. Under conditions in which PKR is activated, the translation initiation factor eIF2α becomes phosphorylated, leading to translational arrest and thereby to a reduction in the rate of Mcl-1 synthesis [14, 28]. As a result, Mcl-1 levels are expected to decline. This scenario has been confirmed recently in in-vitro studies where it was shown directly that treatment of cultured beta cells with the viral double stranded RNA (dsRNA) mimetic, polyinosinic:polycytidylic acid (poly-I:C; which will activate PKR) led to rapid loss of Mcl-1 from the cells [14].
In the present analysis, a similarly striking relationship was observed since, of 152 individual VP1-immunopositive beta cells studied in detail (across eight different cases) all showed loss of immunoreactive Mcl-1 by comparison with those cells immediately adjacent to them. Since we have proved that VP1 production is invariably correlated with PKR induction in beta cells in type 1 diabetes, these results also reveal that the PKR must have become activated under these conditions and that the cells had entered an antiviral state in which bulk protein translation is arrested. As such, all of these features are fully consistent with the concept that viral infection underlies the response. If this is not the case and a specific subset of beta cells are responding to an unrelated stimulus (which, by chance, is identified uniquely, and with high affinity, by the anti-VP1 serum) then this stimulus must also be capable of causing the selective upregulation and activation of PKR in only a small proportion of the available beta cells, thereby driving the loss of Mcl-1. Irrespective of the initiating mechanism, it should be emphasised that the loss of Mcl-1 is likely to render the cells more responsive to the cytotoxic actions of pro-inflammatory cytokines present within the islet milieu and that this could then play a role in their ultimate demise.
On the basis of the data reported here, we suggest that the presence of immunoreactive VP1 within beta cells in human type 1 diabetes is associated with a cellular phenotype that could be indicative of viral infection and enhanced sensitivity to apoptosis. However, definitive studies confirming whether viral infections are causal to beta cell loss in type 1 diabetes are still awaited.
Abbreviations
- eIF2α:
-
Eukaryotic translation initiation factor 2 alpha
- FFPE:
-
Formalin-fixed paraffin-embedded
- ICI:
-
Insulin-containing islet
- IDI:
-
Insulin-deficient islet
- JDRF:
-
Juvenile Diabetes Research Foundation
- Mcl-1:
-
Myeloid cell leukaemia sequence-1
- nPOD:
-
network of Pancreatic Organ donors with Diabetes
- PKR:
-
Protein kinase R
References
Yeung WC, Rawlinson WD, Craig ME (2011) Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ 342:d35
Stene LC, Oikarinen S, Hyoty H et al (2010) Enterovirus infection and progression from islet autoimmunity to type 1 diabetes: the Diabetes and Autoimmunity Study in the Young (DAISY). Diabetes 59:3174–3180
Oikarinen S, Martiskainen M, Tauriainen S et al (2011) Enterovirus RNA in blood is linked to the development of type 1 diabetes. Diabetes 60:276–279
Ghazarian L, Diana J, Simoni Y, Beaudoin L, Lehuen A (2012) Prevention or acceleration of type 1 diabetes by viruses. Cell Mol Life Sci. doi:10.1007/s00018-012-1042-1
Dotta F, Censini S, van Halteren AG et al (2007) Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A 104:5115–5120
Yoon JW, Austin M, Onodera T, Notkins AL (1979) Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med 300:1173–1179
Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG (2009) The prevalence of enteroviral capsid protein VP1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 52:1143–1151
Tracy S, Drescher KM, Jackson JD, Kim K, Kono K (2010) Enteroviruses, type 1 diabetes and hygiene: a complex relationship. Rev Med Virol 20:106–116
Roivainen M, Klingel K (2009) Role of enteroviruses in the pathogenesis of type 1 diabetes. Diabetologia 52:995–996
Campbell-Thompson M, Wasserfall C, Kaddis J et al (2012) Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev. doi:10.1002/dmrr.2316
Foulis AK, Liddle CN, Farquharson MA, Richmond JA, Weir RS (1986) The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia 29:267–274
Ylipaasto P, Kutlu B, Rasilainen S et al (2005) Global profiling of coxsackievirus- and cytokine-induced gene expression in human pancreatic islets. Diabetologia 48:1510–1522
Schulte BM, Lanke KH, Piganelli JD et al (2012) Cytokine and chemokine production by human pancreatic islets upon enterovirus infection. Diabetes 61:2030–2036
Colli ML, Nogueira TC, Allagnat F et al (2011) Exposure to the viral by-product dsRNA or Coxsackievirus B5 triggers pancreatic beta cell apoptosis via a Bim/Mcl-1 imbalance. PLoS Pathog 7:e1002267
Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG (2010) Evidence of increased islet cell proliferation in patients with recent-onset type 1 diabetes. Diabetologia 53:2020–2028
Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG (2009) Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 155:173–181
Willcox A, Richardson SJ, Bone AJ, Foulis A, Morgan NG (2011) Immunohistochemical analysis of the relationship between islet cell proliferation and the production of the enteroviral capsid protein, VP1, in the islets of patients with recent-onset type 1 diabetes. Diabetologia 54:2417–2420
Thomas LW, Lam C, Edwards SW (2010) Mcl-1; the molecular regulation of protein function. FEBS Lett 584:2981–2989
Richardson SJ, Willcox A, Bone AJ, Morgan NG, Foulis AK (2011) Immunopathology of the human pancreas in type-I diabetes. Semin Immunopathol 33:9–21
Chapman NM, Kim KS, Drescher KM, Oka K, Tracy S (2008) 5′ Terminal deletions in the genome of a coxsackievirus B2 strain occurred naturally in human heart. Virology 375:480–491
Kim KS, Tracy S, Tapprich W et al (2005) 5′-Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative-strand viral RNA. J Virol 79:7024–7041
Klingel K, Hohenadl C, Canu A et al (1992) Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc Natl Acad Sci U S A 89:314–318
Tam PE, Messner RP (1999) Molecular mechanisms of coxsackievirus persistence in chronic inflammatory myopathy: viral RNA persists through formation of a double-stranded complex without associated genomic mutations or evolution. J Virol 73:10113–10121
Yin H, Berg AK, Westman J, Hellerstrom C, Frisk G (2002) Complete nucleotide sequence of a Coxsackievirus B-4 strain capable of establishing persistent infection in human pancreatic islet cells: effects on insulin release, proinsulin synthesis, and cell morphology. J Med Virol 68:544–557
Ylipaasto P, Klingel K, Lindberg AM et al (2004) Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 47:225–239
Oikarinen M, Tauriainen S, Honkanen T et al (2008) Analysis of pancreas tissue in a child positive for islet cell antibodies. Diabetologia 51:1796–1802
Klingel K, Sauter M, Bock CT, Szalay G, Schnorr JJ, Kandolf R (2004) Molecular pathology of inflammatory cardiomyopathy. Med Microbiol Immunol 193:101–107
Garcia MA, Gil J, Ventoso I et al (2006) Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev 70:1032–1060
Acknowledgements
This research was performed with the support of the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative type 1 diabetes research project sponsored by the Juvenile Diabetes Research Foundation International (JDRF). Organ procurement organisations partnering with nPOD to provide research resources are listed at www.jdrfnpod.org/our-partners.php.
Funding
The work was supported by funding from the European Union’s Seventh Framework Programme PEVNET (FP7/2007–2013) under grant agreement number 261441 and from a Diabetes Research Wellness Foundation Non-Clinical Research Fellowship to S. J. Richardson.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
SJR was responsible for study conception and design, acquired, analysed and interpreted data and drafted the manuscript. PL acquired and analysed data and edited the manuscript. AJB contributed to the study design and revised the manuscript. AKF collected samples, contributed to study design and conception and revised the manuscript. NGM was responsible for study conception and design, interpreted data and drafted the manuscript. All authors approved the final version.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
(PDF 12 kb)
ESM Table 2
(PDF 11 kb)
ESM Table 3
(PDF 9 kb)
Rights and permissions
About this article
Cite this article
Richardson, S.J., Leete, P., Bone, A.J. et al. Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia 56, 185–193 (2013). https://doi.org/10.1007/s00125-012-2745-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-012-2745-4