Abstract
Purpose
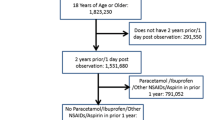
High-dimensional propensity score (hd-PS) adjustment has been proposed as a tool to improve control for confounding in pharmacoepidemiological studies using longitudinal claims databases. We investigated whether hd-PS matching improved confounding by indication in a study of Cox-2 inhibitors (coxibs) and traditional nonsteroidal anti-inflammatory drugs (tNSAIDs) and their association with the risk of upper gastrointestinal complications (UGIC).
Methods
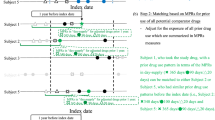
In a cohort study of new users of coxibs and tNSAIDs we compared the effectiveness of these drugs to reduce UGIC using hd-PS matching and conventional propensity score (PS) matching in the German Pharmacoepidemiological Research Database.
Results
The unadjusted rate ratio (RR) of UGIC for coxib users versus tNSAID users was 1.21 [95 % confidence interval (CI) 0.91–1.61]. The conventional PS matched cohort based on 79 investigator-identified covariates resulted in a RR of 0.84 (0.56–1.26). The use of the hd-PS algorithm based on 900 empirical covariates further decreased the RR to 0.62 (0.43–0.91).
Conclusions
A comparison of hd-PS matching versus conventional PS matching resulted in improved point estimates for studying an intended treatment effect of coxibs versus tNSAIDs when benchmarked against results from randomized controlled trials.


Similar content being viewed by others
References
Schneeweiss S, Avorn J (2005) A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol 58(4):323–337
Suissa S, Garbe E (2007) Primer: administrative health databases in observational studies of drug effects—advantages and disadvantages. Nat Clin Pract Rheumatol 3(12):725–732
Schneeweiss S, Gagne JJ, Glynn RJ, Ruhl M, Rassen JA (2011) Assessing the comparative effectiveness of newly marketed medications: methodological challenges and implications for drug development. Clin Pharmacol Ther 90(6):777–790
Schneeweiss S (2007) Developments in post-marketing comparative effectiveness research. Clin Pharmacol Ther 82(2):143–156
Cox E, Martin BC, Van Staa T, Garbe E, Siebert U, Johnson ML (2009) Good research practices for comparative effectiveness research: approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effects using secondary data sources: the International Society for Pharmacoeconomics and Outcomes Research Good Research Practices for Retrospective Database Analysis Task Force Report—Part I. Value Health 12(8):1053–1061
Wooldridge JM (2001) Econometric analysis of cross section and panel data. MIT Press, Cambridge
Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA (2009) High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 20(4):512–522
Schneeweiss S, Rassen J (2011) Re: Confounding adjustment via a semi-automated high-dimensional propensity score algorithm: an application to electronic medical records. Pharmacoepidemiol Drug Saf 20(10):1110–1111
Joffe MM (2009) Exhaustion, automation, theory, and confounding. Epidemiology 20(4):523–524
Pigeot I, Ahrens W (2008) Establishment of a pharmacoepidemiological database in Germany: methodological potential, scientific value and practical limitations. Pharmacoepidemiol Drug Saf 17(3):215–223
Schink T, Garbe E (2010) Representativity of dispensations of non-steroidal anti-inflammatory drugs (NSAIDs) in the German Pharmacoepidemiological Research Database. Pharmacoepidemiol Drug Saf 19:S294
Schink T, Garbe E (2010) Assessment of the representativity of in-patient hospital diagnoses in the German Pharmacoepidemiological Research Database. Pharmacoepidemiol Drug Saf 19:S178–S179
Cattaruzzi C, Troncon MG, Agostinis L, Garcia Rodriguez LA (1999) Positive predictive value of ICD-9th codes for upper gastrointestinal bleeding and perforation in the Sistema Informativo Sanitario Regionale database. J Clin Epidemiol 52(6):499–502
Raiford DS, Perez GS, Garcia Rodriguez LA (1996) Positive predictive value of ICD-9 codes in the identification of cases of complicated peptic ulcer disease in the Saskatchewan hospital automated database. Epidemiology 7(1):101–104
Glynn RJ, Schneeweiss S, Sturmer T (2006) Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 98(3):253–259
Rostom A, Muir K, Dube C, Jolicoeur E, Boucher M, Joyce J, Tugwell P, Wells GW (2007) Gastrointestinal safety of cyclooxygenase-2 inhibitors: a Cochrane Collaboration systematic review. Clin Gastroenterol Hepatol 5(7):818–28, 828
Moore RA, Derry S, Phillips CJ, McQuay HJ (2006) Nonsteroidal anti-inflammatory drugs (NSAIDs), cyxlooxygenase-2 selective inhibitors (coxibs) and gastrointestinal harm: review of clinical trials and clinical practice. BMC Musculoskelet Disord 7:79
Schneeweiss S, Glynn RJ, Avorn J, Solomon DH (2005) A Medicare database review found that physician preferences increasingly outweighed patient characteristics as determinants of first-time prescriptions for COX-2 inhibitors. J Clin Epidemiol 58(1):98–102
Solomon DH, Schneeweiss S, Glynn RJ, Levin R, Avorn J (2003) Determinants of selective cyclooxygenase-2 inhibitor prescribing: are patient or physician characteristics more important? Am J Med 115(9):715–720
Depont F, Fourrier A, Merliere Y et al (2007) Channelling of COX-2 inhibitors to patients at higher gastrointestinal risk but not at lower cardiovascular risk: the Cox2 inhibitors and tNSAIDs description of users (CADEUS) study. Pharmacoepidemiol Drug Saf 16(8):891–900
Laharie D, Droz-Perroteau C, Benichou J et al (2010) Hospitalizations for gastrointestinal and cardiovascular events in the CADEUS cohort of traditional or Coxib NSAID users. Br J Clin Pharmacol 69(3):295–302
Morant SV, Pettitt D, MacDonald TM, Burke TA, Goldstein JL (2004) Application of a propensity score to adjust for channelling bias with NSAIDs. Pharmacoepidemiol Drug Saf 13(6):345–353
MacDonald TM, Morant SV, Goldstein JL, Burke TA, Pettitt D (2003) Channelling bias and the incidence of gastrointestinal haemorrhage in users of meloxicam, coxibs, and older, non-specific non-steroidal anti-inflammatory drugs. Gut 52(9):1265–1270
Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ (2000) Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 343(21):1520–8, 2
Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS (2000) Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 284(10):1247–1255
Toh S, Garcia Rodriguez LA, Hernan MA (2011) Confounding adjustment via a semi-automated high-dimensional propensity score algorithm: an application to electronic medical records. Pharmacoepidemiol Drug Saf 20(8):849–857
Schneeweiss S (2010) A basic study design for expedited safety signal evaluation based on electronic healthcare data. Pharmacoepidemiol Drug Saf 19(8):858–868
Hernan MA, Hernandez-Diaz S (2012) Beyond the intention-to-treat in comparative effectiveness research. Clin Trials 9(1):48–55
Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ (2001) Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 154(9):854–864
Rassen JA, Glynn RJ, Brookhart MA, Schneeweiss S (2011) Covariate selection in high-dimensional propensity score analyses of treatment effects in small samples. Am J Epidemiol 173(12):1404–1413
Sturmer T, Rothman KJ, Avorn J, Glynn RJ (2010) Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution–a simulation study. Am J Epidemiol 172(7):843–854
Cadarette SM, Gagne JJ, Solomon DH, Katz JN, Sturmer T (2010) Confounder summary scores when comparing the effects of multiple drug exposures. Pharmacoepidemiol Drug Saf 19(1):2–9
Rassen JA, Solomon DH, Glynn RJ, Schneeweiss S (2011) Simultaneously assessing intended and unintended treatment effects of multiple treatment options: a pragmatic "matrix design". Pharmacoepidemiol Drug Saf 20(7):675–683
Wei L, Brookhart MA, Schneeweiss S, Setoguchi S (2012) Implications of collider-stratification bias in epidemiologic studies: simulation study. Am J Epidemiol (in press)
Myers JA, Rassen JA, Gagne JJ, Huybrechts KF, Schneeweiss S, Rothman KJ, Joffe MM, Glynn RJ (2011) Effects of adjusting for instrumental variables on bias and precision of effect estimates. Am J Epidemiol 174(11):1213–1222
Acknowledgments
This study was conducted as one subproject of The Safety of Nonsteroidal Anti-inflammatory Drugs (SOS) project (http://www.sos-nsaids-project.org/) which has received funding from the European Community’s Seventh Framework Programme under grant agreement number 223495—the SOS project. The authors are grateful to all statutory health insurance providers that provided data for this study, namely the Allgemeine Ortskrankenkasse (AOK) Bremen/ Bremerhaven, the DAK–Gesundheit, the Techniker Krankenkasse (TK), and the hkk. S. Schneeweiss was supported by grants from the National Library of Medicine (RO1-LM010213), the National Center for Research Resources (RC1-RR028231), and the National Heart Lung and Blood Institute (RC4-HL106373).
Conflict of interest
E. Garbe has received consulting fees by Novartis Pharma GmbH, Bayer AG and TEVA GmbH unrelated to this project and is member of a Scientific Advisory Board of Nycomed, unrelated to this project. S. Schneeweiss is Principal Investigator of the Brigham and Women’s Hospital DEcIDE Center on Comparative Effectiveness Research and the DEcIDE Methods Center, both funded by AHRQ, and of the Harvard–Brigham Drug Safety and Risk Management Research Center, funded by the Federal Drug Administration. S. Schneeweiss is consultant to WHISCON LLC and Booz & Co, and his research is partially funded by investigator-initiated grants from Pfizer, Novartis, and Boehringer–Ingelheim unrelated to the topic of this study. The remaining authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garbe, E., Kloss, S., Suling, M. et al. High-dimensional versus conventional propensity scores in a comparative effectiveness study of coxibs and reduced upper gastrointestinal complications. Eur J Clin Pharmacol 69, 549–557 (2013). https://doi.org/10.1007/s00228-012-1334-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1334-2