Published online Dec 26, 2012. doi: 10.4252/wjsc.v4.i12.120
Revised: October 31, 2012
Accepted: November 17, 2012
Published online: December 26, 2012
Mesenchymal stem cell (MSC) therapy is entering a new era shifting the focus from initial feasibility study to optimization of therapeutic efficacy. However, how MSC therapy facilitates tissue regeneration remains incompletely characterized. Consistent with the emerging notion that secretion of multiple growth factors/cytokines (trophic factors) by MSC provides the underlying tissue regenerative mechanism, the recent study by Bai et al demonstrated a critical therapeutic role of MSC-derived hepatocyte growth factor (HGF) in two animal models of multiple sclerosis (MS), which is a progressive autoimmune disorder caused by damage to the myelin sheath and loss of oligodendrocytes. Although current MS therapies are directed toward attenuation of the immune response, robust repair of myelin sheath likely requires a regenerative approach focusing on long-term replacement of the lost oligodendrocytes. This approach appears feasible because adult organs contain various populations of multipotent resident stem/progenitor cells that may be activated by MSC trophic factors as demonstrated by Bai et al This commentary highlights and discusses the major findings of their studies, emphasizing the anti-inflammatory function and trophic cross-talk mechanisms mediated by HGF and other MSC-derived trophic factors in sustaining the treatment benefits. Identification of multiple functionally synergistic trophic factors, such as HGF and vascular endothelial growth factor, can eventually lead to the development of efficacious cell-free therapeutic regimens targeting a broad spectrum of degenerative conditions.
- Citation: Lee T. Stem cell therapy independent of stemness. World J Stem Cells 2012; 4(12): 120-124
- URL: https://www.wjgnet.com/1948-0210/full/v4/i12/120.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v4.i12.120
Clinical trials of human bone marrow mesenchymal stem cells (MSC) have been initiated for cardiovascular and immune disorders[1,2]. The therapeutic utility of MSC stems in part from the recognition that MSC possess immunomodulatory properties that can be explored for non-autologous (allogeneic) stem cell therapy. Emerging evidence indicates that although MSC exhibit prominent multi-lineage potential, this cellular feature appears to bear little relevance to their therapeutic effects. Instead, the secretion of multiple growth factors/cytokines (trophic factors) by MSC provides the underlying regenerative capacity[3,4]. These findings broach a novel concept of stem cell trophic factor-mediated tissue repair independent of stemness. The work performed by Bai et al[5] studying the therapeutic role of MSC-derived hepatocyte growth factor (HGF) in two animal models of multiple sclerosis (MS) provides yet another convincing evidence for this concept.
MS is a progressive autoimmune and inflammatory disorder caused by damage to the myelin sheath, which is produced by oligodendrocytes and provides the protective covering surrounding nerve cells[6]. The demyelinating process renders axons functionally impaired and susceptible to insult, contributing to physical and cognitive disabilities[7]. Several therapeutic modules are currently in use or under investigation for treating MS[8]. These include oral drugs that disrupt purine and pyrimidine metabolism, modulate sphingosine-1-phosphate receptor, or attenuate oxidative stress. Also used are humanized monoclonal antibodies directed against various immune cell receptors such as CD20, CD25 and CD52, which are intended to rebalance the immune system in favor of tissue regeneration. However, many of these new MS treatments have been found to trigger serious adverse events, and their long-term safety data remain lacking[8]. It should be noted that since these MS therapies are directed toward attenuation of the immune response, robust repair of myelin sheath, which requires a regenerative approach focusing on long-term replacement of the lost oligodendrocytes, may not be effectively achieved. Thus, recent cell-based therapeutic approaches for MSC treatment have received much attention. These cell therapies have used neural stem cells, oligodendrocyte progenitors, and most notably MSC[5,9-11]. This therapeutic strategy is attractive because the adult central nervous system is known to harbor populations of multipotent neural stem cells and oligodendrocyte precursors[12,13] that may be activated by the administered stem cells. In the adult heart, indeed, MSC administration has been found to activate cardiac stem/progenitor cells, contributing to myocardial regeneration[14,15].
The MS therapeutic study demonstrated by Bai et al[5] is based on the use of human MSC-conditioned medium (MSC-CM), which contains a myriad of therapeutically relevant trophic factors. They showed that exposure of neurosphere cultures to MSC-CM resulted in reduced astrocytes and increased oligodendrocyte precursor cells, oligodendrocytes, and neurons. This in vitro finding is mirrored by intravenous infusion of MSC-CM in their MS mice, which was found to reduce functional deficits and accelerate development of oligodendrocytes and neurons in the context of improved remyelination. Further insights came from their biochemical fractionation and characterization of MSC-CM, demonstrating that HGF and its receptor cMet are primarily responsible for the therapeutic benefits. Indeed, both MSC-derived HGF and exogenously supplied HGF promoted regeneration and functional recovery. This conclusion is further strengthened by the use of an HGF-neutralizing antibody and a cMet-blocking antibody, each of which negated the therapeutic effects. Taken together, their studies highlight the critical role of the HGF/cMet axis in MSC therapy for MS and possibly other tissue degenerative conditions.
MSC have long been known to provide stromal support for the growth and differentiation of bone marrow hematopoietic stem cells through cell contact-dependent and -independent mechanisms, the latter of which is mediated by MSC trophic factors[16], which include many hematopoietic growth factors including granulocyte/macrophage colony-stimulating factor (GM/CSF), G-CSF, M-CSF, and interleukin (IL)-7 as well as IL-6-type cytokines[4,17]. Production of these MSC trophic factors can be further enhanced following exposure to Toll-like receptor (TLR) ligands such as lipopolysaccharide and the double-stranded RNA mimetic polyinosinic-polycytidylic acid [poly(I:C)][18,19]. Although TLR activation of the immune system is associated with chronic inflammation, Cole et al[20] demonstrated an unexpected beneficial role for TLR3 in the arterial wall upon systemic administration of poly(I:C). Further, Packard et al[21] found poly(I:C) administration to be protective against cerebral ischemia-reperfusion injury. Since MSC are widely present in vivo and their perivascular origin in multiple human organs appears certain[3,22,23], it is possible that these prophylactic benefits of poly(I:C) may be mediated through its trophic stimulatory effect on the endogenous MSC niches.
Therapeutically, MSC trophic factors can be functionally redundant and synergistic, mediating immune regulation, cytoprotection, host stem cell activation and mobilization, and extracellular tissue remodeling. MSC also interact with cells of both the innate and adaptive immune systems, leading to immunomodulation of their effector functions[24]. The anti-inflammatory property of MSC was indeed highlighted in the study by Bai et al[5], showing that the therapy reduced the levels of multiple inflammatory cytokines and enhanced the levels of multiple anti-inflammatory cytokines produced by the mononuclear cells from the spinal cords. Along this line, Osiris Therapeutics is currently conducting a Phase III trial of MSC in treating several immune disorders such as graft-versus-host disease and Crohn’s disease (http://www.osiris.com). Although how HGF might singly modulate the host immune response remains unclear, the authors speculated that HGF might alter the balance of pro- and anti-inflammatory T cells possibly by influencing the function of dendritic cells, which express cMet and therefore can be modulated by HGF. However, the immunomodulatory function of MSC alone does not appear to lead to effective tissue repair as demonstrated in our recent MSC therapy for the failing hamster heart, which shows that while a low-dose MSC regimen suppressed myocardial inflammation, it failed to promote cardiac repair. On the other hand, the low-dose cell therapy combined with poly(I:C) conditioning of MSC, which amplified HGF and other trophic factors, suppressed inflammation and stimulated myocardial regeneration[18].
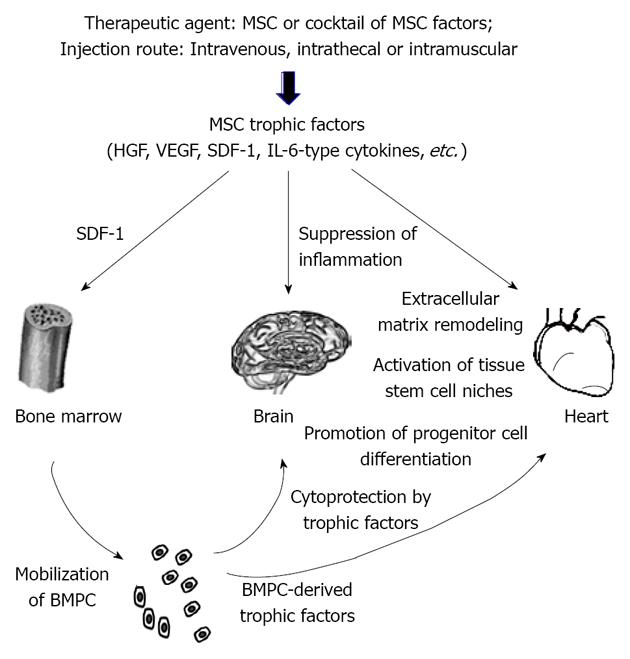
Another important point regarding the therapeutic use of MSC trophic factors is that these soluble mediators typically exhibit a short half-life. Vascular endothelial growth factor (VEGF), for instance, possesses a half-life of about 3 min in circulation[25]. Given a short half-life of HGF[26], the authors raised the question of how this treatment might result in long-term therapeutic benefits. It has previously been found that exogenously administered HGF could result in sustained elevation of endogenous HGF through a positive feedback loop[27]. This finding may not be unexpected given that the growth factor network often exhibits a cross-talk mechanism, enabling induction and amplification of more than one growth factor by another. This trophic cross-talk mechanism has been illustrated in our cardiac therapeutic studies based on intramuscular injection of MSC[4,15]. This MSC therapeutic strategy is coupled to the inherent ability of skeletal muscle to produce beneficial trophic factors in response to exercise and injury[28,29]. Although the injected MSC are trapped in the hamstrings, their trophic actions induce mobilization of bone marrow progenitor cells (BMPC) through the SDF-1/CXCR4 axis and promote increased growth factor levels in the quadriceps, liver, and brain[4,15]. We further demonstrate that the mobilized BMPC are also capable of trophic actions[30], contributing to the systemic increase in trophic factors, which may be explored for MS therapy (Figure 1). Consistent with these preclinical findings, the clinical trials with MS patients revealed similar benefits mediated by either intrathecal or intravenous MSC with no consensus on the best cell delivery route[31]. Note that intravenous infusion of MSC has been adopted for clinical trials of neurodegenerative and heart diseases[2,32]. Although the intravenously infused MSC are largely distributed to the lungs, their trophic actions underlie the observed therapeutic benefits independent of MSC stemness. These findings illustrate the significance of formulating a minimally invasive stem cell delivery approach for patient care.
HGF, like VEGF, also possesses a potent angiogenic function[33]. Administration of HGF, either as a recombinant protein or DNA vector, has been shown to promote angiogenesis without increased vascular permeability or inflammation[34]. Further, HGF can decrease VEGF-mediated leukocyte activation and co-administration of HGF and VEGF more potently promotes angiogenesis than either growth factor alone[35], suggesting that exploring interactions of MSC trophic factors for therapeutic application may be warranted. Coordinated induction of HGF and VEGF following intramuscular administration of MSC is observed in our stem cell and growth factor therapeutic trials for hamster heart failure[4,15,30,36]. This cross activation mechanism may explain why intramuscular injection of VEGF or HGF alone also repairs the failing heart[36,37]. Thus, the beneficial effects observed in the HGF therapy for MS[5] are likely mediated and coordinated by HGF and the many downstream trophic factors induced by HGF. This trophic cascade can also be initiated by MSC-derived IL-6-type cytokines, which signaling through JAK/STAT3 induce HGF, VEGF and many other trophic factors as demonstrated in our MSC therapeutic study[4].
A cautionary note is warranted here because the potent angiogenic function of HGF and VEGF may be associated with a risk of cancer. Indeed, MSC are known to express cancer/testis antigen[38], and MSC-derived VEGF has been reported to promote breast cancer cell migration[39]. However, as noted in a recent review, MSC can also have the potential of diminishing tumor growth, and may be used as “Trojan horses” to deliver anti-cancer therapeutics into the tumor stroma[40]. This controversy may be due to the heterogeneity nature of MSC prepared from differences tissue sources and the use of various experimental models. Interestingly, MSC have been found to be differentially primed by TLR4 and TLR3 ligands to adopt a pro-inflammatory [mesenchymal stem cell (MSC) 1] and anti-inflammatory (MSC2) status, respectively[41]. The MSC1 and MSC2 phenotypes attenuate and promote tumor growth/metastasis, respectively[42]. Along this line, we recently demonstrated that MSC TLR3 activation prominently suppressed tissue inflammation caused by myocyte cell death and promoted myocardial regeneration[18]. These studies thus indicate that the cytokine secretion profile of MSC plays a decisive role in dictating the therapeutic potency and outcome.
In summary, despite encouraging results from numerous preclinical studies, ongoing clinical trials of stem cell therapy have thus far demonstrated moderate and inconsistent benefits[43-45], indicating an urgent need to optimize the therapeutic platform. Identification of multiple functionally synergistic trophic factors, such as HGF and VEGF, can eventually lead to the development of an efficacious cell-free therapeutic regimen. The study by Bai et al[5] paved the way for this logistically attractive approach.
Peer reviewers: Dr. Chau-Hua Chi, Department of Radiation Therapy and Oncology, Shin Kong Wu Ho-Su Memorial Hospital, 95 Wen-Chang Road, Shih-Lin, Taipei, Taiwan, China; Ferenc Sipos, MD, PhD, 2nd Department of Medicine, Semmelweis University, Budapest, Szentkiralyi U 46, H-1088 Budapest, Hungary; Dr. Katrin Schroder, Professor, Institute for Cardiovascular Physiology, Goethe-University Frankfurt, Theodor-Stern-Kai 7, D-60590 Frankfurt, Germany
S- Editor Jiang L L- Editor A E- Editor Xiong L
| 1. | Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 628] [Cited by in F6Publishing: 624] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 2. | Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187-1194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 662] [Cited by in F6Publishing: 646] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 3. | Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1127] [Cited by in F6Publishing: 1134] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 4. | Shabbir A, Zisa D, Lin H, Mastri M, Roloff G, Suzuki G, Lee T. Activation of host tissue trophic factors through JAK-STAT3 signaling: a mechanism of mesenchymal stem cell-mediated cardiac repair. Am J Physiol Heart Circ Physiol. 2010;299:H1428-H1438. [PubMed] [Cited in This Article: ] |
| 5. | Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, Zaremba A, Miller RH. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15:862-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 311] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 6. | Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2859] [Cited by in F6Publishing: 2673] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 7. | Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1514] [Cited by in F6Publishing: 1433] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 8. | Saidha S, Eckstein C, Calabresi PA. New and emerging disease modifying therapies for multiple sclerosis. Ann N Y Acad Sci. 2012;1247:117-137. [PubMed] [Cited in This Article: ] |
| 9. | Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688-694. [PubMed] [Cited in This Article: ] |
| 10. | Busch SA, Horn KP, Cuascut FX, Hawthorne AL, Bai L, Miller RH, Silver J. Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J Neurosci. 2010;30:255-265. [PubMed] [Cited in This Article: ] |
| 11. | Rafei M, Campeau PM, Aguilar-Mahecha A, Buchanan M, Williams P, Birman E, Yuan S, Young YK, Boivin MN, Forner K. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182:5994-6002. [PubMed] [Cited in This Article: ] |
| 12. | Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387-400. [PubMed] [Cited in This Article: ] |
| 13. | Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707-1710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3870] [Cited by in F6Publishing: 3769] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 14. | Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068-14073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 776] [Cited by in F6Publishing: 697] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 15. | Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296:H1888-H1897. [PubMed] [Cited in This Article: ] |
| 16. | Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2398] [Cited by in F6Publishing: 2439] [Article Influence: 174.2] [Reference Citation Analysis (0)] |
| 17. | Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702-709. [PubMed] [Cited in This Article: ] |
| 18. | Mastri M, Shah Z, McLaughlin T, Greene CJ, Baum L, Suzuki G, Lee T. Activation of Toll-like receptor 3 amplifies mesenchymal stem cell trophic factors and enhances therapeutic potency. Am J Physiol Cell Physiol. 2012;303:C1021-C1033. [PubMed] [Cited in This Article: ] |
| 19. | DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential. Mediators Inflamm. 2010;2010:865601. [PubMed] [Cited in This Article: ] |
| 20. | Cole JE, Navin TJ, Cross AJ, Goddard ME, Alexopoulou L, Mitra AT, Davies AH, Flavell RA, Feldmann M, Monaco C. Unexpected protective role for Toll-like receptor 3 in the arterial wall. Proc Natl Acad Sci U S A. 2011;108:2372-2377. [PubMed] [Cited in This Article: ] |
| 21. | Packard AE, Hedges JC, Bahjat FR, Stevens SL, Conlin MJ, Salazar AM, Stenzel-Poore MP. Poly-IC preconditioning protects against cerebral and renal ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2012;32:242-247. [PubMed] [Cited in This Article: ] |
| 22. | Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-313. [PubMed] [Cited in This Article: ] |
| 23. | Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Péault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21:1299-1308. [PubMed] [Cited in This Article: ] |
| 24. | Tyndall A, Walker UA, Cope A, Dazzi F, De Bari C, Fibbe W, Guiducci S, Jones S, Jorgensen C, Le Blanc K. Immunomodulatory properties of mesenchymal stem cells: a review based on an interdisciplinary meeting held at the Kennedy Institute of Rheumatology Division, London, UK, 31 October 2005. Arthritis Res Ther. 2007;9:301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5621] [Cited by in F6Publishing: 5457] [Article Influence: 188.2] [Reference Citation Analysis (0)] |
| 26. | Pan W, Yu Y, Yemane R, Cain C, Yu C, Kastin AJ. Permeation of hepatocyte growth factor across the blood-brain barrier. Exp Neurol. 2006;201:99-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Hayashi S, Morishita R, Higaki J, Aoki M, Moriguchi A, Kida I, Yoshiki S, Matsumoto K, Nakamura T, Kaneda Y. Autocrine-paracrine effects of overexpression of hepatocyte growth factor gene on growth of endothelial cells. Biochem Biophys Res Commun. 1996;220:539-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 488] [Cited by in F6Publishing: 459] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 29. | Rissanen TT, Vajanto I, Hiltunen MO, Rutanen J, Kettunen MI, Niemi M, Leppänen P, Turunen MP, Markkanen JE, Arve K. Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am J Pathol. 2002;160:1393-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Zisa D, Shabbir A, Mastri M, Taylor T, Aleksic I, McDaniel M, Suzuki G, Lee T. Intramuscular VEGF activates an SDF1-dependent progenitor cell cascade and an SDF1-independent muscle paracrine cascade for cardiac repair. Am J Physiol Heart Circ Physiol. 2011;301:H2422-H2432. [PubMed] [Cited in This Article: ] |
| 31. | Karussis D, Kassis I, Kurkalli BG, Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci. 2008;265:131-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277-2286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1006] [Cited by in F6Publishing: 957] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 33. | Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1012] [Cited by in F6Publishing: 1015] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 34. | Shimamura M, Sato N, Oshima K, Aoki M, Kurinami H, Waguri S, Uchiyama Y, Ogihara T, Kaneda Y, Morishita R. Novel therapeutic strategy to treat brain ischemia: overexpression of hepatocyte growth factor gene reduced ischemic injury without cerebral edema in rat model. Circulation. 2004;109:424-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Min JK, Lee YM, Kim JH, Kim YM, Kim SW, Lee SY, Gho YS, Oh GT, Kwon YG. Hepatocyte growth factor suppresses vascular endothelial growth factor-induced expression of endothelial ICAM-1 and VCAM-1 by inhibiting the nuclear factor-kappaB pathway. Circ Res. 2005;96:300-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Zisa D, Shabbir A, Mastri M, Suzuki G, Lee T. Intramuscular VEGF repairs the failing heart: role of host-derived growth factors and mobilization of progenitor cells. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1503-R1515. [PubMed] [Cited in This Article: ] |
| 37. | Komamura K, Tatsumi R, Miyazaki J, Matsumoto K, Yamato E, Nakamura T, Shimizu Y, Nakatani T, Kitamura S, Tomoike H. Treatment of dilated cardiomyopathy with electroporation of hepatocyte growth factor gene into skeletal muscle. Hypertension. 2004;44:365-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Cronwright G, Le Blanc K, Götherström C, Darcy P, Ehnman M, Brodin B. Cancer/testis antigen expression in human mesenchymal stem cells: down-regulation of SSX impairs cell migration and matrix metalloproteinase 2 expression. Cancer Res. 2005;65:2207-2215. [PubMed] [Cited in This Article: ] |
| 39. | De Luca A, Lamura L, Gallo M, Maffia V, Normanno N. Mesenchymal stem cell-derived interleukin-6 and vascular endothelial growth factor promote breast cancer cell migration. J Cell Biochem. 2012;113:3363-3370. [PubMed] [Cited in This Article: ] |
| 40. | Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F. Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11-19. [PubMed] [Cited in This Article: ] |
| 41. | Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. [PubMed] [Cited in This Article: ] |
| 42. | Waterman RS, Henkle SL, Betancourt AM. Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PLoS One. 2012;7:e45590. [PubMed] [Cited in This Article: ] |
| 43. | Malliaras K, Kreke M, Marbán E. The stuttering progress of cell therapy for heart disease. Clin Pharmacol Ther. 2011;90:532-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Allison M. Genzyme backs Osiris, despite Prochymal flop. Nat Biotechnol. 2009;27:966-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Tyndall A. Successes and failures of stem cell transplantation in autoimmune diseases. Hematology Am Soc Hematol Educ Program. 2011;2011:280-284. [PubMed] [Cited in This Article: ] |