Article Text
Abstract
Introduction Pregnancy entails both pancreatic adaptations with increasing β-cell mass and immunological alterations in healthy women. In this study, we have examined the effects of pregnancy on β-cell function and immunological processes in long-standing type 1 diabetes (L-T1D).
Research design and methods Fasting and stimulated C-peptide were measured after an oral glucose tolerance test in pregnant women with L-T1D (n=17) during the first trimester, third trimester, and 5–8 weeks post partum. Two 92-plex Olink panels were used to measure proteins in plasma. Non-pregnant women with L-T1D (n=30) were included for comparison.
Results Fasting C-peptide was detected to a higher degree in women with L-T1D during gestation and after parturition (first trimester: 64.7%, third trimester: 76.5%, and post partum: 64.7% vs 26.7% in non-pregnant women). Also, total insulin secretion and peak C-peptide increased during pregnancy. The plasma protein levels in pregnant women with L-T1D was dynamic, but few analytes were functionally related. Specifically, peripheral levels of prolactin (PRL), prokineticin (PROK)-1, and glucagon (GCG) were elevated during gestation whereas levels of proteins related to leukocyte migration (CCL11), T cell activation (CD28), and antigen presentation (such as CD83) were reduced.
Conclusions In summary, we have found that some C-peptide secretion, that is, an indirect measurement of endogenous insulin production, is regained in women with L-T1D during pregnancy, which might be attributed to elevated peripheral levels of PRL, PROK-1, or GCG.
- pregnancy
- C-peptide
- immunomodulation
- diabetes mellitus, type 1
Data availability statement
Data are available on reasonable request. Data will be made available on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Pregnancy entails both pancreatic and immunological adaptations.
β-Cell mass increase during pregnancy in healthy women.
WHAT THIS STUDY ADDS
Some endogenous insulin production is regained during pregnancy in women with long-standing type 1 diabetes.
Pregnant women with type 1 diabetes also developed a peripheral immunoprofile characterized by altered leukocyte migration and maturation and activation of T cells and antigen-presenting cells.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
By investigating physiological effects of pregnancy on women with T1D, it might be possible to imitate a β-cell trophic environment and develop new treatments for individuals with T1D.
Introduction
Pregnancy entails several physiological adaptions, which are essential to prevent rejection of the conceptus and ensure successful fetal development. There is a gradual increase in insulin secretory response in healthy women throughout gestation, while there is a decline in peripheral insulin sensitivity only during the last trimester.1 2 Augmented insulin secretion appears to be independent of insulin sensitivity,2 indicating that the pancreas undergoes intrinsic adaptions during pregnancy. In 1978, Van Assche et al discovered that the fraction of endocrine tissue within the pancreas and the percentage of β-cells within islets increase in pregnant women.3 A more recent study revealed that a greater fractional β-cell area is attributed to an increased density of small islets and dispersed β-cells among exocrine tissue.4
It is still debated whether improved function, reduced apoptosis, enhanced proliferation, or neogenesis of β-cells leads to augmented insulin secretion, but it is evident that metabolic alterations coincide with elevated serum levels of prolactin (PRL), human placental lactogen (hPL), 17-β-estradiol, and progesterone (PG).5 Indeed, several studies have shown that these hormones directly affect β-cell function and expansion. In healthy women, lower serum levels of PRL during early pregnancy were an independent predictor of reduced β-cell function, whole-body insulin sensitivity, and glucose tolerance during gestation and the postpartum period.6 A comprehensive in vitro study showed that stimulation of human islets with either PRL or hPL increases the cumulative insulin secretion, although hPL had a greater potency than PRL.7 The inferior potency of PRL might be explained by a low receptor expression on human β-cells that is also connected to a limited proliferative response during pregnancy.8 In contrast to humans, evidence from pancreas-specific knockout mice clearly shows how important PRL is for the increase in β-cell mass and glucose-dependent insulin secretion.9
The effects of pregnancy on pancreatic endocrine function in type 1 diabetes (T1D) remain equivocal. Most women improve their glycemic control, but up to 45% of pregnant women with T1D experience in parallel a higher rate of severe hypoglycemia.10 Interestingly, the daily insulin requirement for women with T1D is reduced during the first trimester compared with preconception and later trimesters.11 By measuring fasting or random C-peptide concentrations, it has been suggested that insulin secretion in pregnant women with long-standing T1D (L-T1D) is gradually regained.12–14 These studies have later been questioned by a study with 10 individuals, where no gestational alterations in plasma C-peptide were identified after mixed meal tolerance tests.15 However, the above-mentioned studies did not include control cohorts and none of them used ultrasensitive C-peptide assays.
Pregnancy in healthy women involves sequential immunological alterations, which are strictly regulated by steroid and placental hormones.16 Conversely, there are few comparative immunological studies between healthy women and individuals with L-T1D.17 18 It is essential to deduce if any regained insulin secretion is connected to β-cell recovery, β-cell neogenesis, or immunomodulation in addition to determining which hormones regulate the physiological adaption. Thus, this prospective study aimed to examine longitudinal effects on β-cell function and peripheral protein levels in pregnant women with L-T1D. Non-pregnant (NP) women with L-T1D were included to compare selected variables. Fasting and stimulated C-peptide, hormones, and proteins related to cell regulatory and immunological processes were measured in blood samples obtained during gestation and 2 months after parturition.
Research design and methods
Study design and eligibility
Pregnant women with L-T1D (n=15) were recruited to a prospective cohort study at Uppsala and Örebro University Hospitals, Sweden. Pregnant patients were eligible for the study if they had been diagnosed with T1D for >10 years at conception and were <12 weeks of gestation. Research visits were performed on three occasions (described as mean value (range)): (1) gestation week 11.6 (10–13), (2) gestation week 34.4 (33–36) and (3) 6.2 (5–8) weeks post partum. Two women had a consecutive pregnancy shortly after the first delivery and were followed once again according to the study protocol. Therefore, they were considered as two new biological samples (total sample size, n=17). All women except two were breast feeding at the postpartum visit. Samples from a single time-point acquired from NP women with L-T1D (n=30) were used as a control for selected variables.
Clinical chemistry and oral glucose tolerance test
All visits were performed in the morning (08:00–10:00 hours) after an overnight fast. Peripheral venous blood was collected for analyses at Clinical Chemistry Laboratory, Uppsala University Hospital, and Department of Laboratory Medicine, Örebro University Hospital according to clinical routine. Triglycerides were not analyzed for patients included at Örebro University Hospital, rendering 11 samples for statistical analysis. EDTA-plasma was stored at −70°C prior to analysis of C-peptide and circulating proteins.
At each visit, an oral glucose tolerance test (OGTT) was performed. The OGTT comprised ingestion of a 50 g glucose solution (250 mL), instead of the prevalent 75 g glucose load, and blood was collected at T0=before ingestion, T1=10 min, T2=30 min, T3=60 min, T4=90 min, and T5=120 min. Long-acting insulin was administered as per routine but no rapid-acting insulin was administered prior to the OGTT.
Enzyme-linked immunosorbent assay
An ultrasensitive ELISA (Mercodia; Sweden, product no. 10-1141-01) was used to measure fasting and stimulated C-peptide. The detection limit for the assay was 2.5 pmol/L (0.0076 µg/L). All samples from both pregnant and NP women were stored at −70°C for <12 months prior to C-peptide measurements.
Proximity extension assay
Protein analysis was performed in undiluted plasma by multiplex proximity extension assay (PEA) at Olink Proteomics, Uppsala, Sweden. Two validated 92-plex panels, Olink CELL REGULATION and Olink IMMUNE RESPONSE (online supplemental tables 1 and 2), were used to measure analytes associated with biological processes such as lymphocyte activation, cytokine-mediated signaling, apoptosis, and differentiation.
Supplemental material
Supplemental material
Data analysis and statistics
The ratio between triglycerides and high-density lipoprotein-cholesterol was used to calculate insulin resistance at each visit, where a cut-off value of 1.65 was applied.19 Patients were considered positive for autoantibodies if anti-GAD was >5 IU/mL and anti-IA2 >7 kU/L. Area under the curve (AUC) for C-peptide values during OGTTs was calculated by the trapezoid rule. Statistical analyses were conducted with GraphPad Prism V.9 and IBM SPSS Statistics V.27. Sharpio-Wilk and Kolmogorov-Smirnov tests were applied to determine the data distribution of clinical variables. C-peptide data were transformed to 10-logarithmic values when a Gaussian distribution was not followed, and normality tests were again performed to evaluate transformed data. Two-tailed unpaired Welch’s t-test or two-tailed unpaired Mann-Whitney U test was used to compare data between pregnant participants and NP participants. A linear mixed effects model, followed by the Benjamini-Hochberg method for false discovery rate, were applied to compare longitudinal changes in clinical variables. For non-parametric longitudinal data, Friedman test and uncorrected Dunn’s post hoc test were used. The χ2 test was applied to compare frequencies of categorical variables, such as C-peptide detectability and autoantibody positivity. A significance level of α=0.05 was defined for all tests.
For analysis of protein levels, data processing was performed in Olink NPX Manager. By using predetermined correction factors for each analyte, adjusted Cq-values were inverted and transformed into a relative arbitrary unit called Normalized Protein eXpression (NPX). NPX is expressed on a logarithmic scale (log2), meaning that a difference of +1 NPX corresponds to a concentration doubling. Statistical analyses were conducted with RStudio V.4.0.0. Principal component analysis (PCA) was applied to identify outliers and clusters. Two-sided unpaired Welch’s t-test was used to compare protein levels between NP individuals and both trimesters, separately. A linear mixed effects model was applied to assess longitudinal changes in protein levels between trimesters and the postpartum period. A maximum likelihood approach was chosen for parameter estimation, where visits and individuals constituted fixed and random effects, respectively. An unstructured covariance matrix was assumed. The Benjamini-Hochberg method was applied for correction of multiple testing in the model, and a false discovery rate <0.05 was considered significant. A network analysis was performed on the online platform STRING V.11.0 (STRING: functional protein association networks (string-db.org)). A full network type was chosen to identify both functional and physical protein interactions, the interaction score was set at a high confidence level (0.700), and edges illustrate the strength of data support (confidence). A network was considered to have significantly more interactions than expected at a protein–protein interaction (PPI) enrichment p value <0.05. UniProtKB (UniProt) and The Human Protein Atlas V.19.3 (The Human Protein Atlas) were implemented to obtain in-depth information on protein function and tissue expression. Explorations were made using UniProt numbers specified in Olink panels.
Results
Demographic data for study subjects
The pregnant participants were in average 3.2 years older than the NP women (table 1).
Demographic data of patients with long-standing type 1 diabetes (L-T1D)
The presence of GAD and IA2 autoantibodies in peripheral blood was similar between pregnant and NP women and did not change along the study. However, the number of IA2-positive women increased by one at each study visit. Total cholesterol, LDL, and triglyceride levels increased considerably to attain a peak at late gestational ages, which was ensued by a reduction after parturition. Nevertheless, total cholesterol and LDL remained more abundant 2 months post partum compared with NP women and during the first trimester. Due to decreased plasma triglyceride levels, the degree of insulin resistance was lower in pregnant women at first trimester compared with NP individuals with L-T1D. In parallel with elevated daily insulin doses, the insulin resistance increased during the third trimester. Interestingly, insulin doses were overall lower for women 2 months after parturition than in the NP group despite no differences in insulin resistance. The glycemic control improved during pregnancy and persisted after delivery in comparison with NP women with L-T1D. Glycated hemoglobin (HbA1c) was even lower during the third trimester than at early gestational ages. PRL was only measured in serum samples obtained from pregnant women. PRL levels increased during pregnancy and peaked at third trimester prior to a decline after parturition, although PRL remained elevated compared with the first trimester.
β-Cell function in women with L-T1D was recovered during gestation, but decreased after parturition
To assess the effect of pregnancy on β-cell function, fasting and stimulated C-peptide were measured. First, it was evaluated whether C-peptide becomes measurable during pregnancy in comparison with NP individuals. The frequency of women with L-T1D having detectable fasting C-peptide was consistently higher during gestation and after parturition (first trimester: 64.7%, third trimester: 76.5%, and 2 months post partum: 64.7%) than for NP women (26.7%, figure 1A). Despite an apparent variance within the NP group, the absolute concentration of fasting C-peptide was also higher in pregnant women than in NP individuals with L-T1D (figure 1B, table 1). This difference between the groups remained 2 months after parturition.
Fasting and glucose-induced C-peptide levels in pregnant women with diabetes. Peripheral venous blood was collected from pregnant individuals with long-standing type 1 diabetes (L-T1D, n=17) during three visits: first trimester (1T), third trimester (3T), and 2 months post partum (2PP). For comparison, plasma samples from non-pregnant women with L-T1D (NP, n=30) were used. A reduced oral glucose tolerance test (OGTT) was performed to assess the effect of pregnancy on glucose-induced insulin secretion. C-peptide in plasma samples obtained during fasting and the OGTT was measured by an ultrasensitive ELISA. (A) The number of individuals with non-detectable (ND) and detectable (D) fasting C-peptide was calculated. A χ2 test was applied to compare frequencies for both outcomes in each group. (B) The absolute concentration of fasting C-peptide was estimated between the NP group and pregnant women with L-T1D. Data were transformed to 10-logarithmic values. Dots and lines represent individual samples and median values, respectively. Two-sided Mann-Whitney U test was applied for statistical analyses between each visit and the NP group. Longitudinal changes in (C) absolute fasting C-peptide concentrations, (D) total C-peptide secretion, and (E) peak C-peptide concentrations during OGTT are shown in 10-logarithmic values. Differences between peak and fasting C-peptide values during OGTT are visualized as (F) absolute change and (G) percental change. Calculations were made on non-transformed data. Fasting C-peptide was set to 100% to calculate percental change during OGTT (dashed line). Graphs show median values and 95% CIs for each visit. Non-parametric Friedman test with uncorrected Dunn’s test for familywise α-threshold was applied for statistical analysis. *P<0.05, **p<0.01, ***p<0.001. AUC, area under the curve.
At a group level, fasting C-peptide levels did not change between gestational ages and the postpartum period (figure 1C). However, at the individual level most women with L-T1D had a rise in fasting C-peptide during pregnancy followed by a reduction or stabilization after parturition. Few participants had a nadir of fasting C-peptide that was recovered in the postpartum period. Both AUC and peak C-peptide after a glucose load increased during pregnancy (figure 1D,E). Moreover, the difference between the highest C-peptide concentrations and fasting values was enhanced between first and third trimesters (figure 1F). The glucose-induced insulin secretion declined after parturition, although the percental response during OGTT did not seem to be reduced (figure 1G). All C-peptide and glucose data from the OGTT is presented in online supplemental table 4.
Supplemental material
The circulating protein levels of pregnant women with L-T1D is dynamic, but few functional clusters were related to the maternal immune system and potential modulators of β-cell function.
Plasma proteins associated with cellular and immunological processes were measured to investigate how they varies during gestation in women with T1D. Seventy-seven of 85 samples for Olink CELL REGULATION and 78 of 85 samples for Olink IMMUNE RESPONSE passed the quality control (online supplemental figure 1), rendering 16 samples from the first trimester, 15 samples from the third trimester, and 15 samples from the postpartum period for analysis. All 184 analytes were included in the analysis, as they were detected in ≥25% of the samples. First, a two-dimensional projection of the multivariate dataset and samples was computed by unsupervised PCA to identify outliers and potential clusters. Neither outliers nor clusters were found among the samples, meaning that the study groups had similar protein concentrations at a global level (online supplemental figure 2).
Supplemental material
Supplemental material
To investigate whether the protein composition diverges during pregnancy from a ‘T1D-background’, plasma samples from each trimester were compared with the NP individuals. Only 16 analytes were differently abundant between women during early gestation and NP participants with L-T1D, whereas the discrepancy between later gestational ages and the control cohort was enhanced (online supplemental table 3). A supervised network analysis was then performed to investigate functional associations between diverging analytes (n=49). A network comprising 11 edges between 16 nodes was identified, which could be divided into five clusters (PPI value=0.0008, figure 2A). Only 35% of the network was associated with maternal immune processes as visualized by functional enrichments. The largest cluster comprised several nodes that had a single interaction with the pleiotropic cytokine interleukin (IL)-6. Levels of IL-6 were higher in women with L-T1D during the third trimester than in the NP group (figure 2B). The chemokine CCL11 (eotaxin) was continuously less abundant in the pregnant women than in the NP individuals. Fibroblast growth factor (FGF)-21 was functionally connected to glucagon (GCG) signaling, and plasma levels of this factor were lower at first trimester compared with NP women with L-T1D. IL-6 was directly associated with amphiregulin (AREG), which is a protein that mediates PG production in placental tissue.20 21 AREG was also higher during the whole gestational period than in the NP group. Cytoskeleton-associated protein (CKAP)4 was associated with neutrophil-mediated immunity and was more abundant during pregnancy than in the NP participants. CKAP4 was connected to members of the C-type lectin (CLEC) domain family. CLEC4C was less abundant, while CLEC4D and CLEC7A were more abundant during the third trimester than in the NP women. A cluster classified as endocrine modification was identified, which comprised prokineticin (PROK)-1 and GCG (figure 2A and C). PROK-1 was more abundant throughout gestation in comparison with the NP participants, while plasma levels of GCG were only higher during the third trimester. A cluster related to antigen endocytosis was identified, where CLEC4A and lymphocyte antigen (LY)75 were reduced in plasma from women with L-T1D at third trimester (figure 2A and D). Two minor clusters related to diabetic complications (figure 2E) and neutrophil-associated hydrolases (figure 2F) were also found.
Supplemental material
Network analysis of divergent proteins between pregnant and non-pregnant women with diabetes. Peripheral venous blood was collected from women with long-standing type 1 diabetes (L-T1D) at two visits during pregnancy: first trimester (1T, n=16) and third trimester (3T, n=15). For comparison, plasma samples from non-pregnant women with L-T1D (NP, n=30) were used. Proximity extension assay was employed to measure 184 analytes in plasma. A network analysis was performed to assess functional and physical interactions between 49 diverging proteins (16 proteins for 1T vs NP, 47 proteins for 3T vs NP). (A) Nodes corresponding to 49 proteins (abbreviated names) were formed, and 11 edges (black lines) were identified in the network. This network had a PPI enrichment p value <0.001 and an average local clustering coefficient=0.265. Edge thickness indicates the confidence level of each interaction: high (0.700) and maximum (0.900) scores. Filled nodes represent proteins with a known or predicted three-dimensional structure. Functional enrichments within the network were computed, where colors represent annotations that could describe nodes connected by edges. Connected nodes were classified based on enrichments in the network and protein database explorations: (B) interleukin (IL)-6 governed pathways, (C) endocrine modification, (D) antigen processing, (E) diabetic complications, (F) and enzyme activity. Protein levels in plasma are shown as Normalized Protein eXpression (NPX) that is an arbitrary unit expressed on the log2-scale. Analytes that were detected below their lower limits of detection are indicated with dashed lines ([beta-galactosidase [GLB1]: 1.6 NPX, ARSB: −0.009 NPX). Dots represent individual samples and filled lines indicate mean values for the groups. Two-sided unpaired Welch’s t-test with adjustment for multiple testing was applied for statistical analysis, *q<0.05, **q<0.01, ***q<0.001, and ****q<0.0001. AREG, amphiregulin; CKAP4, cytoskeleton-associated protein; CLEC, C-type lectin; GCG, glucagon; PROK, prokineticin.
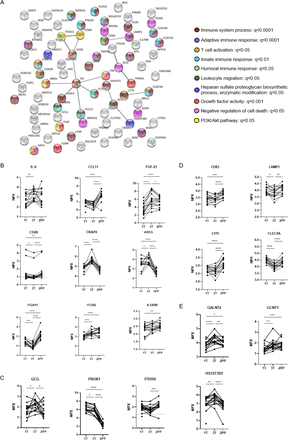
A linear mixed model was also applied to assess variations in the protein composition of individuals with L-T1D during pregnancy and after parturition. Temporal differences were identified for 79 analytes, where the largest contrast was observed between the third trimester and 2 months post partum (online supplemental table 3). The supervised network analysis showed that only 19 proteins formed 15 interactions at high confidence level, but this network was non-random (PPI value=0.0007, figure 3A). Only 35% of all nodes were related to the immune system, as visualized by functional enrichments within the network. IL-6 was again connected to CCL11, FGF-21, AREG, and CKAP4, but to some extent with different functional enrichments. IL-6 increased from first to third trimester, as mirrored by the comparison with the NP group, and remained stable after parturition (figure 3B). The nadir of CCL11 during gestation disappeared 2 months after parturition and became similar to levels found in the NP women. FGF-21 was associated with growth factor activity and cell death inhibition in this network (figure 3A), which increased in plasma until the third trimester and then declined during the postpartum period (figure 3B). AREG and CKAP4 peaked at third trimester prior to a decline after parturition. In contrast to the previous network analysis, IL-6 was connected to the T cell co-receptor CD28 and indirectly to integrin proteins. Soluble CD28 was continuously reduced during pregnancy, but it increased again after parturition to higher levels than at early gestational ages. PROK-1 and GCG still formed a functional interaction within this network, although parathyroid hormone-related peptide receptor was included (figure 3A). PROK-1 levels were constantly high in pregnant women with L-T1D prior to a nadir 2 months post partum, while GCG peaked at third trimester prior to a decrease after parturition (figure 3C). CLEC4A, LY75, CD83, and lysosomal associated membrane protein (LAMP)3 were classified into one cluster, as they are functionally related to antigen-presenting cells (APC). In addition, these four proteins had similar longitudinal changes in women with L-T1D: suppressed during pregnancy before a rebound after childbirth (figure 3D). Three enzymes catalyzing protein glycosylation, indirectly regulating leukocyte migration and extravasation, were predominant in plasma samples from the third trimester (figure 3A and E).
Network analysis of divergent proteins in pregnant women with diabetes. Plasma samples were obtained from pregnant women with long-standing type 1 diabetes (L-T1D) during three visits: first trimester (1T, n=16), third trimester (3T, n=15), and 2 months post partum (2PP, n=15). Proximity extension assay was employed to measure 184 analytes in plasma. A network analysis was performed to assess functional and physical interactions between 79 diverging proteins. (A) Nodes corresponding to all 79 proteins (abbreviated names) were formed, and 15 edges (black lines) were identified in the network. This network had a PPI enrichment p value <0.001 and an average local clustering coefficient=0.194. Edge thickness indicates the confidence level of each interaction: high (0.700) and maximum (0.900) scores. Filled nodes represent proteins with a known or predicted three-dimensional structure. Functional enrichments within the network were computed, where colors represent annotations that could describe nodes connected by edges. Connected nodes were classified based on enrichments in the network and protein database explorations: (B) IL-6 governed pathways, (C) endocrine modification, (D) antigen presentation pathway, (E) and enzyme activity. Protein levels in plasma are shown as Normalized Protein eXpression (NPX) that is an arbitrary unit expressed on the log2-scale. Analytes that were detected below their lower limits of detection are indicated with dashed lines (CD28: 0.98 NPX, PTH1R: 1.3 NPX). Dots and lines represent individual samples and repeated measures at the specified visits, respectively. A linear mixed effects model with maximum likelihood approach was chosen for parameter estimation. Visits and individuals constituted fixed and random effects, respectively. An unstructured covariance matrix was assumed. False discovery rate by the Benjamini-Hochberg method was applied for correction of multiple testing in the model, *q<0.05, **q<0.01, ***q<0.001, and ****q<0.0001. AREG, amphiregulin; CKAP4, cytoskeleton-associated protein; CLEC, C-type lectin; GCG, glucagon; PROK, prokineticin.
Conclusions
Although individuals with T1D improve their glycemic control during pregnancy, it is not evident whether it is solely a consequence of improved motivation in combination with frequent advice on metabolic control from healthcare personnel or if some regain of insulin production could also contribute. Studies that have measured fasting or induced C-peptide, a surrogate marker for insulin production, in pregnant individuals are conflicting. Prospective studies investigating gestational alterations within the peripheral immune system in T1D are also lacking. Thus, we aimed to examine the longitudinal effect of pregnancy on endogenous insulin production in women with L-T1D. We also used a high-throughput multiplex immunoassay to analyze proteins related to hormonal, cell regulatory, and immunological processes, which allowed us to examine possible associations with C-peptide secretion, that is, an indirect measurement of β-cell. function We demonstrate that insulin secretion was recovered during gestation but decreased slowly after parturition. Despite that the analyzed plasma protein levels in pregnant women with L-T1D was dynamic, a limited number of analytes might be related to improved β-cell function.
Our study shows that the detectability and levels of C-peptide were higher in pregnant women with T1D, supporting the idea that pregnancy induces a long-standing recovery of β-cell function. This is in line with previous studies on only fasting C-peptide,12 13 but differs from a study mainly evaluating stimulated C-peptide.15 Smaller sample size, lower assay sensitivity, and suboptimal conditions for mixed meal tolerance test can explain the differences from Murphy et al.15 A recent large-scale study by Meek et al have demonstrated that a rise in C-peptide during late pregnancy could also be attributed to an increased fetal insulin production and transfer of C-peptide to maternal blood.22 However, in our study we found that many of the women retained elevated C-peptide levels also after parturition which would argue against a transfer of fetal C-peptide in the women included in our study. Although insulin resistance and daily insulin doses were elevated during the last trimester, most likely due to anabolic processes,23 the improved HbA1c during and beyond pregnancy in women with T1D correlates with increased β-cell function.
Several mechanisms within the pancreas that lead to enhanced insulin secretion in healthy pregnant women have been suggested. It is known that β-cells are a heterogeneous population, comprising a proliferative subset and quiescent cells with a mature phenotype.24 In addition, it has recently been established that individuals with L-T1D retain a small proportion of β-cells.25 Our study does not provide evidence or mechanisms for either reduced apoptosis, proliferation or neogenesis of β-cells in pregnant women with T1D, but we are able to reveal interesting associations between increased C-peptide secretion, that is, suggestive of improved β-cell function, and circulating proteins. Higher serum levels of PRL in parallel with improved glucose-induced insulin secretion during pregnancy suggest that PRL could have modulated secretory pathways within residual β-cells, although without inducing proliferation.7 26 PROK-1 is another analyte that was specifically increased in plasma during gestation. PROK-1 is secreted by cells in the placenta that promotes angiogenesis and capillary fenestration only in endocrine glands.27 28 Data from in vitro studies using normal and tumor cells show that PROK-1 and its receptor are present within the pancreas, which can stimulate proliferation and reduce apoptosis of endothelial cells.29 30 It was interesting that both IL-6 and GCG were elevated during later gestational ages, as animal studies have provided insights to the relationship between IL-6, GCG, and insulin secretion. IL-6 appears to have a direct effect on pancreatic α-cells and promotes GCG secretion.31 32 During non-inflammatory conditions, IL-6 stimulated enzymatic processing of GCG into glucagon-like peptide (GLP)-1 that led to improved insulin secretion and glycemic control.32 Moreover, GLP-1 is one of the factors that have been found to stimulate β-cell proliferation, mass expansion, and insulin production in pregnant mice.33 However, it should be noted that apart from GLP-1 a number of other factors have been linked to β-cell proliferation in experimental studies, including for instance serotonin34 and hepatocyte growth factor.35
Pregnant women with L-T1D also displayed dynamic changes of plasma proteins related to immunological processes. Lower levels of CCL11 between the first and third trimesters are in line with findings in healthy women reporting that CCL11 and other pro-inflammatory chemokines are reduced throughout gestation before rebounding after parturition.36 37 It was interesting that CCL11 was even less abundant in pregnant women than in the NP group. In accordance with our network analysis, women with L-T1D can consequently develop a gestation-associated shift from leukocyte migration and humoral immune responses. A reduction of soluble CD28 during pregnancy might be beneficial for glycemic control in T1D, as an increased peripheral concentration of CD28 has been connected to elevated disease activity in other autoimmune diseases.38–40 Moreover, it has been reported that healthy women who experienced spontaneous abortions had increased CD28 expression in decidual tissues in parallel with an elevated frequency of IFN-γ+ T cells.41 These studies infer that T cell activation, survival, or function is impaired in women with T1D during pregnancy, which might have beneficial effects on non-placental tissues such as pancreatic islets. According to the Uniprot database, expression of CLEC4A, CLEC4C, LY75, CD83, and LAMP3 defines the maturation, activation, or function of monocytes and dendritic cells. APC play a critical role for a successful pregnancy, and imbalances in subset ratios appear to cause obstetric complications in healthy women.42 Thus, reduced levels of these proteins in plasma from pregnant women with L-T1D might reflect a suppressed APC maturation, function (affecting downstream lymphocyte activation), or tissue recruitment. Also, a recent study have found that the number of regulatory T-cells are higher in women with L-T1D who increase in C-peptide during pregnancy when compared with those without rise in C-peptide.43 Altogether, our results support the idea that gestation-related immunomodulation seen in healthy women can indeed be also developed in individuals with T1D.
The inclusion of NP women with L-T1D enabled us to compare metabolic and protein variables with a control group. This cohort was however recruited from a previous study that did not employ OGTT44 and samples were only collected at one time-point, precluding an assessment of differences in glucose-induced insulin secretion. Regarding our method, PEA is a sensitive discovery-oriented technology that facilitated analysis of well-known and exploratory proteins associated with pregnancy. Olink panels are designed based on information from bioinformatic databases, such as Uniprot, Gene Ontology, and DisGeNET. The panels are thus derived from computational predictions, knowledge transfer between organisms, and aggregated data from previous experiments. This circumstance entails some issues. First, several analytes that were detected in plasma are secreted or expressed within several tissues and cell types. Second, all analytes have different expression patterns that span from ubiquitous to stimuli-induced. These features make it complicated to draw definite conclusions about origin and functional consequences of divergent plasma proteins and hence its implications for pancreatic islets. As a significant amount of bioinformatic data are based on gene analyses, it is also important to query the relevance of ascribed protein annotations.
This study shows that women with L-T1D regain C-peptide secretion during gestation, which is an indirect measurement of β-cell function that might be attributed to elevated peripheral levels of PRL, PROK-1, or GCG via GLP-1. As indicated by PEA, pregnant individuals with L-T1D also developed a peripheral immunoprofile characterized by altered leukocyte migration and maturation, activation, or function of T cells and APC. Consequently, enhanced insulin production by residual β-cells might be further protected by a gestation-associated immunomodulation. By investigating physiological effects of pregnancy on women with T1D, it might be possible to imitate a β-cell trophic environment and develop new treatments for individuals with T1D.
Data availability statement
Data are available on reasonable request. Data will be made available on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study was approved by the Regional Research Ethical Committee in Uppsala (Dnr 2013/273) and was conducted in consistency with the Declaration of Helsinki. All participants gave their written informed consent prior to inclusion in the study.
Acknowledgments
We acknowledge Olink Proteomics in Uppsala, Sweden, for the protein analysis and biostatistician services as well as Mercodia in Uppsala for the assistance with C-peptide analyses. We also appreciate the advisory role of Lars Valter, epidemiological statistician at Forum Östergötland, Sweden. Research nurses Rebecka Hilmius and Karin Kjellström, Uppsala University Hospital, Sweden, are gratefully acknowledged for their skilled assistance. Lastly, we would like to thank all women and new mothers who participated in this study.
References
Footnotes
DE and LM are joint first authors.
Contributors DE and P-OC were responsible for study conception, patient recruitment and clinical data collection. JC-C and ES recruited patients and collected clinical data. DE acquired clinical and proteomic data. DE calculated the total insulin secretion. LM calculated the insulin resistance, analyzed clinical and proteomic data, made the network analyses, and interpreted data. LM wrote the manuscript, which was revised by DE and P-OC. Manuscript submission was approved by all authors. P-OC is the guarantor of this manuscript.
Funding This study was supported by the Swedish Research Council, Barndiabetesfonden, Diabetesfonden, the Novo Nordisk Foundation, the Swedish Society for Medical Research, and the Regional research council in Uppsala-Örebro.
Disclaimer Funders were not involved in the study design, acquisition, analysis, and interpretation of data, or writing of this article.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.