Article Text
Abstract
Recent studies have associated non-alcoholic fatty liver disease (NAFLD) with impaired cardiac function. However, patients with type 2 diabetes mellitus (T2DM), a high-risk group for left ventricular diastolic dysfunction (LVDD), were not analyzed as an independent study population. A systematic review was conducted to identify all published clinical trials using the PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure, and Wanfang databases from inception to September 14, 2022. Observational studies that reported echocardiographic parameters in T2DM patients with NAFLD compared with those without NAFLD were included for further selection. The Agency for Healthcare Research and Quality checklist was used to appraise the study quality. Ten observational studies (all cross-sectional in design) comprising 1800 T2DM patients (1124 with NAFLD, 62.4%) were included. We found that T2DM patients with NAFLD had a significantly lower E/A ratio, higher peak A velocity, higher E/e’ ratio, lower e’ velocity, greater left atrial maximum volume index, and greater left ventricular mass index than non-NAFLD patients. These findings reinforced the importance of NAFLD being associated with an increased risk of LVDD in the T2DM population, and NAFLD may be a sign of LVDD in patients with T2DM.
PROSPERO registration number
CRD42022355844.
- diastolic dysfunction
- non-alcoholic fatty liver disease
- diabetes mellitus, type 2
- meta-analysis
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
The association between non-alcoholic fatty liver disease (NAFLD) and impaired cardiac functions has been demonstrated. However, patients with type 2 diabetes mellitus (T2DM) were not analyzed as an independent population in those studies. This association might be influenced by the synergistic effects of NAFLD and T2DM, which exacerbate myocardial injury.
WHAT THIS STUDY ADDS
Changes in glycaemic control and the presence of T2DM are crucial for altering cardiac dysfunction. This systematic review and meta-analysis found that NAFLD is associated with an increased risk of LVDD in the T2DM population.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
When NAFLD is diagnosed in patients with diabetes, routine echocardiography should be performed to assess the cardiac diastolic impairment. NAFLD may be a sign of LVDD in patients with T2DM.
Introduction
According to the Diabetes Atlas of the International Diabetes Federation, the number of people with diabetes mellitus (DM) worldwide in 2021 is 537 million, which represents an increase of 74 million (16%) compared with that in 2019, highlighting an astonishing rise in DM prevalence worldwide.1 Type 2 diabetes mellitus (T2DM) is the most common type of DM and has a profound impact on the cardiovascular system.2 Cardiovascular disease (CVD), particularly heart failure (HF), is the leading cause of death in patients with T2DM, which is thought to be due to concurrent coronary artery disease, hypertension, and/or ischemic cardiomyopathy.3 4 However, several studies have shown that myocardial injury can occur independently of traditional CVD risk factors.5–7 This condition was defined as diabetic cardiomyopathy (DCM), which primarily leads to left ventricular diastolic dysfunction (LVDD) through increased chamber stiffness and hypertrophy with high resting tension in the early phase, and eventually leads to HF with preserved ejection fraction (HFpEF).2 8 9 Thus, the identification and prevention of CVD in patients with T2DM have become an essential issue.
A systematic review of 80 studies has shown that the prevalence of non-alcoholic fatty liver disease (NAFLD) is one of the most pressing health issues globally, reaching 55.5% in patients with T2DM, indicating two times the higher prevalence in the T2DM population than that in the general population.10 11 Furthermore, accumulating evidence indicates that NAFLD is strongly associated with an increased risk of cardiac dysfunction, and LVDD is the main phenotype.12 13 Owing to the insidious onset of LVDD, cardiac structure and function changes might occur earlier than the symptoms of CVD.14 Two-dimensional echocardiography using tissue Doppler imaging is the most convenient and reliable diagnostic method for evaluating subclinical changes in cardiac function. In 2016, the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) suggested using routine echocardiographic assessments to evaluate LV diastolic function.15
Previous meta-analyses have demonstrated an association between NAFLD and impaired cardiac systolic and diastolic functions.13 However, patients with T2DM, a high-risk group for NAFLD and LVDD that might lead to HF over time, were not analyzed as an independent population in those studies. This association might be influenced by the synergistic effects of NAFLD and T2DM, which exacerbate myocardial injury. To our knowledge, there has been no systematic review of the association between NAFLD and LVDD in patients with T2DM. Therefore, our meta-analysis aimed to (1) elucidate whether NAFLD is associated with the risk of LVDD via echocardiographic parameters, a relatively objective and detailed assessment, in the T2DM population and (2) explore whether NAFLD is a sign of LVDD in terms of early detection of the risk of developing HFpEF and improving prognosis in patients with T2DM.
Materials and methods
Search strategies and selection criteria
We conducted our systematic review and meta-analysis in accordance with the Meta-analysis of Observational Studies in Epidemiology group guidelines.16 A comprehensive search strategy was performed to establish our datasets from five electronic databases: PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure, and Wanfang. The search was conducted from its inception to September 14, 2022. Using PubMed as an example, medical subject heading terms (eg, NAFLD, DM) and free-text terms (eg, non-alcoholic steatohepatitis, non-alcoholic fatty liver, diabetes) were used in the literature search, and Boolean search terms (OR, AND) were used to merge different knowledge domains. The detailed PubMed retrievals are listed in online supplemental table S1. Because the retrieval rules were similar, retrieval strategies of other databases only needed to be modified based on the retrieval formula of PubMed. Additional relevant documents were manually searched from the reference lists of the included studies, meeting abstracts, and updated systematic reviews to avoid omission. Our study has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42022355844.
Supplemental material
Two investigators (XZ and QZ) independently screened the abstracts or full texts of each publication for eligibility, and any discrepancies were settled through consultation with other authors to obtain more precise data selection. Publications fulfilling the following criteria were included in our meta-analysis: (1) study design: observational studies that were published with no language restriction; potential studies in non-English were translated via translation software; and only full-length original research studies that were published in peer-reviewed journals were selected. (2) Population: all included participants were adults (≥18 years old) with a definite diagnosis of T2DM. (3) Comparison: studies comparing LVDD-related parameters between T2DM patients with NAFLD and those without NAFLD, and the diagnostic criteria of NAFLD were based on invasive liver biopsy or non-invasive diagnostic modalities. (4) Outcomes: the assessment of LVDD from ASE/EACVI guidelines used more than one of the following echocardiographic parameters: peak E velocity (E), peak A velocity (A), mean mitral annular diastolic velocity (e’), E/e’ ratio, E/A ratio, deceleration time (DT), LV mass index (LVMI), and left atrial maximum volume index (LA, LAVImax). We excluded studies if (1) they were duplicated; (2) they did not specify the type of DM or only defined NAFLD using blood-based markers; and (3) the same population data were published in different articles.
Quality assessment and data extraction
The risk of bias and methodological quality of each study were evaluated using the Cross-Sectional/Prevalence Study Quality (CSSQ), which is a validated quality scale with a range of 0–11 points for cross-sectional studies, and it was recommended by the Agency for Healthcare Research and Quality (USA). The CSSQ scale contains 11 items, each with three options: “yes” (scored 1), “no” (scored 0), and “unclear” (scored 0), and the maximum CSSQ score of 11 indicates the highest level of study quality.17
Data from the included articles were extracted directly and separately using a standardized data form in duplicate. Specifically, the main content of the data included study characteristics (authorship, study publication year, study location, study design, and quality assessment score), patient details (sample size, sex proportion, mean age, baseline CVD, and diagnostic modalities for NAFLD), and main outcomes (echocardiographic parameters related to LVDD). For continuous variables, we extracted the mean and SD. If the data in the original study did not conform to the normal distribution, we used the sample size, median, and quartile provided by the authors to estimate the mean and SD using mathematical formulas.18 19 Two investigators (XZ and QZ) evaluated and extracted eligible articles independently, and their work was double-checked by a third investigator.
Statistical analysis
The meta-analysis was performed by the meta R package using R Studio (R V.4.2.0). To determine the effect size of each echocardiographic parameter and evaluate the changes in diastolic function in T2DM patients with and without NAFLD, the weighted mean differences (WMD) were calculated along with 95% CIs. Statistical significance was considered for p-value <0.05. Statistical heterogeneity was assessed using Cochrane’s Q test and I2, and the value of I2 indicated whether there was heterogeneity among studies (<25%, 25%–75%, and >75% represent low, moderate, and substantial heterogeneity, respectively).20 All echocardiography parameters were analyzed using random-effects models with a two-tailed statistical comparison, and forest plots were used to visualize the results. Sensitivity analysis with fixed-effects models was conducted to confirm reliability. Additionally, we recalculated the pooled effect estimates by removing each study to further detect the source of heterogeneity. Publication bias was quantitatively examined by conducting Egger’s regression test and visually assessed using funnel plots for outcomes with a sufficient sample size (n≥10), considering p-value <0.05, a statistically significant bias.21
Results
Study identification
Based on our designed search strategy, 412 records were initially identified, and duplicates were removed from the six records. After screening the titles and abstracts, 34 original research articles published in peer-reviewed journals were included for full-text review. Ten studies were included in our meta-analysis based on the previously mentioned eligibility criteria. A detailed flowchart of the search strategy is shown in figure 1.
Flowchart of the search strategy and study selection. NAFLD, non-alcoholic fatty liver disease; T2DM, type 2 diabetes mellitus.
Study characteristics
Ten observational studies comprising 1800 T2DM participants, of which 1124 (62.4%) T2DM with NAFLD were included.22–31 We assessed the risk of bias in all eligible studies based on the CSSQ checklist, and all studies achieved scores of 7–9 points (see online supplemental table S2). These observational studies were conducted based on a cross-sectional design, with three retrospective reports and seven prospective reports. In total, five studies were conducted in China (50%), two were conducted in Korea (20%), and one study was performed in Italy, India, and Canada (10%). Overall, the included population was predominantly Asians (80%). As for the diagnostic modalities and evaluation of NAFLD, liver transient elastography was used in two studies, and liver (abdominal) ultrasound was used in eight studies. The main characteristics of the studies are listed in table 1.
Characteristics of the included studies and participants
NAFLD and the risk of LVDD in patients with T2DM
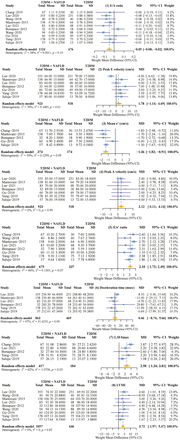
We conducted our meta-analysis using the eight most commonly indicated echocardiographic parameters. T2DM patients with NAFLD had a significantly lower E/A ratio (WMD: −0.05 (95% CI −0.08 to −0.02); p<0.01), higher peak A velocity (WMD: 2.12 (95% CI 0.11 to 4.14); p<0.05), higher E/e’ ratio (WMD: 2.10 (95% CI 1.72 to 2.49); p<0.01), lower e’ velocity (WMD: −1.37 (95% CI −1.82 to −0.91); p<0.01), greater LAVImax (WMD: 2.58 (95% CI 1.34 to 3.81); p<0.01), and greater LVMI (WMD: 3.72 (95% CI 1.97 to 5.47); p<0.01) than non-NAFLD patients (table 2, figure 2). In general, compared with that in patients without NAFLD, the risk of incident LVDD was markedly increased in T2DM patients with NAFLD.
Detailed results of the difference in echocardiographic outcomes
The forest plot for each diastolic-dysfunction-related echocardiographic parameters. e’, mean mitral annular diastolic velocity; LAVImax, left atrial maximum volume index; LVMI, left ventricular mass index; MD, mean difference; NAFLD, non-alcoholic fatty liver disease; T2DM, type 2 diabetes mellitus.
Sensitivity analysis
The pooled results of all the sensitivity analyses are shown in online supplemental figure S1. We used fixed-effect models to recalculate the WMD of each diastolic-dysfunction-related echocardiographic parameter, which is consistent with our meta-analysis, indicating that our results are not affected by mathematical model selection. We then calculated the overall effect estimates after omitting each study individually to identify potential sources of heterogeneity. The peak E velocity changed significantly after omitting the paper published by Lee et al in 2020. However, the results of the other seven indicators, including E/A and E/e’ ratio, were unchanged with moderate or low heterogeneity. In particular, the LVMI and peak A velocity showed no heterogeneity (I2=0). The risk of publication bias was not detected based on the funnel plot (see online supplemental figure S2) or statistical asymmetry test (p-value=0.6181 for Egger’s regression test). Overall, our meta-analysis results were reliable.
Discussion
In the present meta-analysis based on published articles, we synthesized data from 10 cross-sectional designed studies with a total of 1800 participants and proved that T2DM patients with NAFLD were more likely to suffer from LVDD than those without NAFLD. Recently, a comprehensive meta-analysis of 41 studies involving 33 891 participants showed that NAFLD is associated with systolic and diastolic impairments.13 The authors found that hemoglobin A1c was a predictor of reduced LV ejection fraction (LVEF), and age was a predictor of diastolic dysfunction due to a higher E/e ratio, which showed their insight into glucose metabolism indicators affecting cardiac function. However, changes in glycemic control and the presence of T2DM are crucial for altering cardiac dysfunction.2
Hence, it is necessary to confirm the association between NAFLD and diastolic dysfunction in the T2DM population, and the assessment of diastolic function according to ASE/EACVI guidelines should have more dimensions.15 The prevalence of DM is a severe issue in developing countries, and China is the largest developing country with a considerable number of individuals suffering from DM.32 To obtain comprehensive data, we did not restrict the language of the articles to English. To our knowledge, this is the first published meta-analysis to confirm the association between NAFLD and LVDD risk in a T2DM population using echocardiographic parameters.
Specifically, the results of our study showed that, compared with the control group, the NAFLD group had a significantly lower E/A ratio, higher peak A velocity, lower e’, higher E/e’ ratio, greater LAVImax, and greater LVMI, which collectively indicated an increased risk of LVDD in T2DM patients with NAFLD. The E/A ratio, as an effective, specific, and reproducible transmitral flow index, is often used in multiple large-scale clinical studies to define LVDD.33–35 All of our included articles used this index to evaluate cardiac diastolic function, and the results of our meta-analysis also showed a significant statistical difference. However, the E/A ratio has a U-shaped relationship with LV diastolic function, and an abnormally high value can be observed in advanced LVDD.15
Therefore, to avoid difficulty in distinguishing normal diastolic function from pseudonormal diastolic function, it is indispensable to assess LVDD in combination with other parameters, including tissue Doppler indices. Overall, the more abnormal the parameters, the more suggestive the diagnosis of LVDD.36 The E/e' ratio is an age-dependent Doppler parameter that is positively correlated with the LV filling pressure. Although 8 to 14 is the gray zone of the E/e' ratio, and whether the pressure is elevated with other parameters must be determined with caution, it is highly specific when the ratio is greater than 14.15
The left atrium functions as a blood reservoir, conduit, and booster pump during cardiac circulation and is closely related to LV early diastolic function. In addition to the cumulative effect of increasing LV filling pressure over time, prominent evidence of LVDD diagnosis was also observed in LA volume.15 37 As one of the parameters recommended by ASE for evaluating LVDD, LAVImax was found to be significantly greater in the T2DM with NAFLD group in our study. In addition, LV mass has been found to be an early marker of LVDD and a powerful predictor of increased HF risk.38 39 Although LVM is an indicator used to measure cardiac structure, it is still advocated as an essential supplementary index to evaluate diastolic dysfunction in ASE/EACVI guidelines.15 Moreover, DM directly leads to increased LVM and consequently affects diastolic function, which is also a process in DCM.40 41 It is believed that the increase in LVM in patients with T2DM is a clinical practice issue of great need to be addressed.38 Sensitivity analysis showed that the results of parameters with statistical differences were stable, indicating the reliable effect of our meta-analysis result that NAFLD is associated with an increased risk of LVDD. Interestingly, the underlying mechanism of this phenomenon is worth speculation.
Multiple well-known epidemiological trials have confirmed the link between DM and HF,5 42 and the presence of DM could lead to a 33% higher risk of hospitalizations for HF (adjusted OR: 1.33; 95% CI: 1.18 to 1.50).43 DM might be a traditional CVD risk factor that mediates the occurrence of HF. Otherwise, it could be the only perpetrator of DCM. Despite the fact that DCM is an abnormality of cardiac structure and function, of which diagnosis requires the exclusion of coronary artery disease and hypertension, the natural augmentation of DM on ventricular dysfunction is persistent. Therefore, DCM is an indispensable factor in HF research to describe the vulnerability of the myocardium to dysfunction.2 44
DM-related glucose and lipid metabolism disorders can induce DCM, triggering oxidative stress and inflammation via different signaling pathways.2 Hyperglycemia plays an essential role in the pathogenesis of DCM, activating a series of pathological changes or processes such as lipotoxicity, cardiomyocyte apoptosis, and myocardial fibrosis.45–47 Under diabetic conditions, the heart is exposed to an environment of excessive fatty acids, which results in the accumulation of metabolic intermediates and by-products, eventually manifesting as cardiac steatosis.48–50 Thus, DCM can also be regarded as a lipid-storage disease.
Although there is currently no consensus on the pathological mechanisms of DCM, DM can independently induce an absolute or relative increase in LV mass, resulting in LV hypertrophy that progressively affects diastolic ventricular filling, isovolumetric relaxation, and LV end-diastolic pressure.40 41 This initial phase, accompanied by subtle symptoms, has the potential to progress to HFpEF and eventually lead to HF with reduced ejection fraction (HFrEF), another phenotype of HF dominated by systolic dysfunction.51 HFpEF accounts for a large proportion, with approximately 30%–50% of HF cases in DM,47 which have more severe clinical outcomes than HFrEF, manifesting as increased LV mass and reduced LV diastolic distensibility.52 53
The European Society of Cardiology proposed that the diagnosis of HFpEF should meet the requirements of LVEF greater than 50% with evidence of diastolic dysfunction.54 LVDD results from impaired myocardium relaxation ability leading to decreased left ventricular flexibility, which is considered the main contributing factor for the development of HFpEF and the first sign of cardiac function abnormality in patients with T2DM.55 56
Lipid metabolism is a major contributor to ventricular function. NAFLD is a metabolic-dysfunction-related liver disease that occurs without excessive alcohol consumption (women: less than 20 g/day; men: less than 30 g/day), including steatosis, fatty infiltration, inflammation (non-alcoholic steatohepatitis), fibrosis, and ultimately cirrhosis.57 In the past few years, it has been increasingly recognized that NAFLD can also affect the extrahepatic system, and its relationship with T2DM is bidirectional. T2DM might accelerate the development of NAFLD from steatosis to non-alcoholic steatohepatitis, cirrhosis, and even hepatocellular carcinoma, whereas the presence of NAFLD would induce poor glycemic control in patients with T2DM.58 Emerging evidence suggests that NAFLD is a hallmark of ectopic fat accumulation in the myocardium, and a significant positive correlation of fat content could be observed between intramyocardial and intrahepatic.25 59 60 Myocardial lipid accumulation is due to increased free fatty acids, which influence LV diastolic function.61 62 Furthermore, sufficient findings point out that the dysregulated secretion of proinflammatory cytokines and hepatokines in the progression of NAFLD promotes inflammation and oxidative stress in the myocardial tissue and manifests as cardiac dysfunction.63 However, the exact pathophysiological mechanisms that connect NAFLD to LVDD remain unclear. NAFLD is likely a part of the pathogenesis associated with DM augmentation of diastolic dysfunction, leading to abnormal cardiac structure and function rather than just a simple accompanying symptom or a target organ of this cardiac pathological alteration.
Most of the patients with DM who present with diastolic dysfunction have an insidious clinical asymptomatic period, and many of them remain under no assessment and treatment of LV diastolic function because symptom onset rarely occurs in the initial stage. Identifying T2DM patients with NAFLD at an early stage can provide early warning of their risk of developing LVDD and even HFpEF. Our study demonstrated that NAFLD is associated with LVDD risk in the T2DM population. Although causality cannot be established owing to the lack of longitudinal study evidence, it is still a relatively early warning approach for screening patients at higher risk and improving their prognosis, as myocardial injury precedes the development of cardiac dysfunction that can be observed by a reliable diagnostic technique.
Admittedly, our meta-analysis has some limitations. First, all of these included studies were cross-sectional in design, which inevitably led to the inability to demonstrate the causality of NAFLD with an increased risk of LVDD in the T2DM population. Second, owing to the small number of original studies, stratification analysis could not be performed in our study. Third, several echocardiographic parameters in our study had moderate heterogeneity; therefore, we used random-effects models instead of fixed-effects models, which could produce more reliable estimates.64 In addition, most of the individuals in the original studies were registered in Asia, so the interpretation of the results needs to be done with caution, and further high-quality studies are required.
Conclusions
This systematic review and meta-analysis found that NAFLD is associated with an increased risk of LVDD in the T2DM population. When NAFLD is diagnosed in patients with diabetes, routine echocardiography should be performed to assess the cardiac diastolic impairment. This first step needs to be further confirmed in prospective cohort studies. Identifying serum or plasma biomarkers with high specificity that could more accurately reflect the risk of LVDD in patients with T2DMshould be pursued in the future.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors SW designed the study protocol and wrote the first draft of the manuscript. XZ and QZ extracted the data, performed analyses, and interpreted the data. BZ and LZ contributed to the polishing and revision of the manuscript, supervised the study, and resolved all discrepancies. All authors revised the manuscript critically and approved the final version of the manuscript. SW is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding This research was funded by the Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (CI2021A01605); the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-D-202001); the National Key Research and Development Program of China (2019YFC1709904); the National Natural Science Foundation of China (82104835); and the China Postdoctoral Science Foundation (2021M693542).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.