Article Text
Abstract
Introduction Healthy diet and physical activity (PA) are essential for preventing type 2 diabetes, particularly, a combination of diet and PA. However, reports on interaction between PA and diet, especially from large epidemiological studies, are limited. We investigated the effect of interaction between PA and macronutrient intake on hemoglobin A1c (HbA1c) levels in the general population.
Research design and methods We conducted a cross-sectional study of 55 469 men and women without diabetes who participated in the baseline survey of the Japan Multi-Institutional Collaborative Cohort Study. A self-administered questionnaire ascertained PA and macronutrient intake (carbohydrate, fat, and protein). Multiple linear regression analyses were performed to adjust for confounding variables and examine the interactions. In addition, we conducted a longitudinal study during a 5-year period within a subcohort (n=6881) with accelerometer-assessed PA data.
Results Overall, PA had a weak inverse association (β=−0.00033, p=0.049) and carbohydrate intake had a strong positive association (β=0.00393, p<0.001) with HbA1c. We observed a tendency of interactions between PA and carbohydrate or fat intake, but not protein intake, on HbA1c levels after adjusting for age, sex, study area, total energy intake, alcohol consumption, smoking, and medication for hypertension or hypercholesterolemia (Pinteraction=0.054, 0.006, and 0.156, respectively). The inverse associations between PA and HbA1c level were more evident in participants with high-carbohydrate (or low-fat) intake than in participants with low-carbohydrate (or high-fat) intake. Although further adjustment for body mass index slightly attenuated the above interactions (Pinteraction=0.098 for carbohydrate and 0.068 for fat), the associations between PA and HbA1c level in stratified analyses remained unchanged. Similar associations and interactions were reproduced in the longitudinal study.
Conclusions The present results suggest that the effect of PA on HbA1c levels is modified by intake of macronutrient composition.
- glycated hemoglobin
- exercise
- nutrients
- epidemiology
Data availability statement
Data are available upon reasonable request. Details can be found on the J-MICC Study website (http://www.jmicc.com/).
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Significance of this study
What is already known about this subject?
Physical activity (PA) is associated with lower hemoglobin A1c (HbA1c) level.
Macronutrient (carbohydrate, fat, and protein) intake also affects HbA1c level.
What are the new findings?
This large-scale population-based cross-sectional study revealed the effect of interactions between PA and macronutrient intake on HbA1c level.
The inverse associations between PA and HbA1c level were more evident in participants with higher carbohydrate or lower fat intake than in participants with lower carbohydrate or higher fat intake.
Moreover, these interactions and associations were reproduced in the longitudinal study.
How might these results change the focus of research or clinical practice?
This study shows that the control of HbA1c could become more effective and sustainable by implementing an optimized diet and PA according to possible interactions as observed in this study.
Introduction
In 2019, the number of people with diabetes was estimated to be 463 million globally, and the prevalence continues to increase.1 2 Diabetes causes various complications and significant socioeconomic burden; it also affects health and quality of life. Hemoglobin A1c (HbA1c) is a good indicator of average blood glucose levels over previous 2–3 months and is used to diagnose diabetes. Studies have reported that people with type 2 diabetes have higher HbA1c levels long before the onset of diabetes than those of people without diabetes.3 4
Type 2 diabetes accounts for >90% of all diabetes cases and is caused by complex factors, such as socioeconomic, environmental, and genetic factors, among which lifestyle factors, such as diet and physical activity (PA), are considered as the most important.5 Lifestyle modification is useful for type 2 diabetes prevention and treatment. It has been reported that lifestyle interventions, including diet, reduce the incidence of type 2 diabetes.6 7 Exercise is also effective for the prevention and treatment of type 2 diabetes.8 PA in daily life is important, as well as exercise, and there is evidence showing the effects of PA on the prevention and treatment of type 2 diabetes.9
Diet and PA are also closely related in terms of energy homeostasis, intervention studies on type 2 diabetes are often conducted with a combination of diet and PA. In two systematic review reports, the effect of intervention with a combination of diet and PA on blood glucose control was consistently positive, whereas the effect of diet or PA intervention alone was not consistent.10 11 Energy is mainly produced from macronutrients (carbohydrate, fat, and protein), but some studies have reported no relationship between the intake of carbohydrate or fat and the onset of type 2 diabetes,12 13 and there is no clear evidence to determine the most effective macronutrient intake composition for blood glucose control.5 14 Moreover, there is evidence showing that PA intervention alone had no effect in reducing the risk of developing type 2 diabetes.10 In contrast, a study demonstrated that the effect of exercise intervention on blood glucose levels could be modified according to the intake of macronutrient composition.15 Based on these findings, it is considered that the interaction between diet and PA is an important factor for type 2 diabetes prevention and treatment.
However, evidence on the effect of interaction between diet and PA on blood glucose or HbA1c levels is limited, and no large epidemiological study has been conducted. We hypothesized that the effect of PA on HbA1c levels could be modified by the status of nutritional intake. To explore our hypothesis, we conducted this study to investigate the effect of interaction between PA and macronutrient (carbohydrate, fat, and protein) intake on HbA1c levels in the Japanese general population.
Research design and methods
Study participants
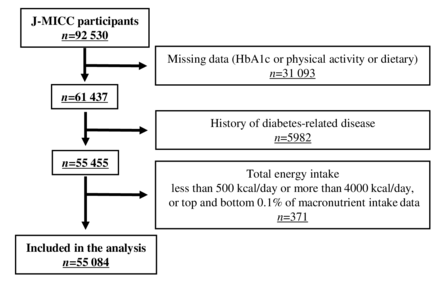
Participants were recruited by the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study, which comprised a large cohort that has been followed up to explore the relationship between lifestyle, genotype, blood composition, and the risk of developing various diseases. The details of the J-MICC Study have been described in a previous report.16 17 The J-MICC Study was initiated in 2005, and participants aged 35–69 years were voluntarily enrolled from the following 13 sites in Japan: Aichi, Chiba, Fukuoka, Iga, Kagoshima, Kyushu-KOPS, Kyoto, Okazaki, Sakuragaoka, Saga, Shizuoka-Daiko, Takashima, and Tokushima subcohorts. In the present cross-sectional study, we used data (V.20201218) obtained from 92 530 participants nationally registered between 2004 and 2008. In the analysis, we excluded 37 446 participants who had missing data on HbA1c level (n=31 093), a history of diabetes-related diseases, such as diabetes, stroke, heart disease, liver disease, and cancer (n=5982), and a dietary total energy intake of <500 or >4000 kcal/day or outliers corresponding to the top and bottom 0.1% of macronutrient intake data (n=371). Consequently, data from 55 084 participants aged 35–69 years were included in the analysis (figure 1), among whom some had missing data on alcohol consumption (n=69), smoking (n=193), medication for hypertension or hypercholesterolemia (n=45), and body mass index (BMI) (n=12).
Flow chart of the process for selecting study participants. HbA1c, hemoglobin A1c; J-MICC, Japan Multi-Institutional Collaborative Cohort.
In the longitudinal study, we used data obtained from 8454 participants who completed the baseline survey and the second survey after 5 years in a subcohort of the J-MICC Study (J-MICC Saga).18 In the analysis, we excluded 1573 participants who had missing data on HbA1c level or PA (n=704), a history of diabetes-related diseases at baseline (n=832), and a dietary total energy intake of <500 or >4000 kcal/day or outliers corresponding to the top and bottom 0.1% of macronutrient intake data (n=37). Consequently, 6881 participants were included in the longitudinal analysis.
Questionnaire and measurements
Self-administered questionnaires were used to collect data on PA, eating habits, alcohol consumption, smoking, medication, and disease history. Height and weight were measured to the nearest 0.1 cm and 0.1 kg, respectively, and BMI was calculated from the measured height and weight. Systolic blood pressure and diastolic blood pressure were measured by anthropometry at the study site. HbA1c level was measured using latex aggregation immunoassay at each study center using blood samples of participants, and the measurement results were collected. The measured HbA1c value (Japan Diabetes Society (JDS) value) was converted into the National Glycohemoglobin Standardization Program (NGSP) equivalent value using the following formula: HbA1c (NGSP [%])=1.02×HbA1c (JDS [%])+0.25.19 Moreover, HbA1c value (International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) value) was calculated using the following formula: HbA1c (IFCC [mmol/mol])=10.93×HbA1c (NGSP [%])−23.50.20 In the present study, the analysis was conducted using HbA1c (NGSP (%)). In the longitudinal study, although the data collection and measurement methods were the same as above, objective PA was additionally assessed using the accelerometer.
PA assessment
PA was evaluated in terms of metabolic equivalents (METs) from the questionnaire data, where MET values of ≥3 were assessed as PA. In the present study, PA was defined as total PA, including daily and leisure-time activity. Participants reported the average time spent per day for each of the following daily activities: heavy physical work time (assigned MET intensity: 4.5 METs) and walking time (3.0 METs). Average time was assigned as follows: none (0), <1 hour/day (0.5), 1–<3 hours/day (2.0), 3–<5 hours/day (4.0), 5–<7 hours/day (6.0), 7–<9 hours/day (8.0), 9–<11 hours/day (10.0), and ≥11 hours/day (12.0). PA (MET×hour/day) in daily life was estimated by multiplying the average time and assigned MET intensity in each activity. For leisure-time activity, participants reported the frequency and average duration for each of the activities as follows: low-intensity PA (such as walking and golf) (assigned MET intensity: 3.4 METs), moderate-intensity PA (such as jogging and swimming) (7.0 METs), and high-intensity PA (such as marathon running and martial arts) (10 METs). Frequency was assigned for daily average as follows: none (0), 1–3 times/month (0.1), 1–2 times/week (0.2), 3–4 times/week (0.5), and 5–6 times/week (0.8). Average duration was assigned for average hours as follows: <30 min (0.3), 30 min–<1 hour (0.8), 1–<2 hours (1.5), 2–<3 hours (2.5), 3–<4 hours (3.5), and ≥4 hours (4.5). The PA (METs×hour/day) of leisure-time activity was estimated by multiplying the frequency, average duration, and assigned MET intensity in each activity. In the present study, PA was calculated by summing the daily and leisure-time activities.
In the longitudinal study, to measure PA, the participants were instructed to attach a single-axis accelerometer (Life-corder, Suzuken Co, Nagoya, Japan) during all waking hours (except when bathing and sleeping) for 10 days, as described in a previous report.21 PA was evaluated for MET values ≥3, and PA (METs×hour/day) was calculated as a sum of mathematical products between corresponding METs and the time spent at corresponding intensity levels.
Dietary assessment
A validated short Food Frequency Questionnaire (FFQ) was used for dietary assessment,22–25 and the total energy and macronutrient (carbohydrate, fat, and protein) intake were estimated. Participants answered the average intake of 46 foods over the past year. The amounts and frequencies were confirmed for three staple foods (rice, bread, and noodles) eaten at breakfast, lunch, and supper. The frequency for the staple foods was assigned as follows: almost none (0), 1–3 times/month (0.1), 1–2 times/week (0.2), 3–4 times/week (0.5), 5–6 times/week (0.8), and daily (1). The staple food eaten at each meal was counted as bowls/day for rice and noodles or slices (or rolls)/day for bread, and the values obtained for three meals were summed. For the remaining 43 foods, only frequency options were provided, as follows (assigned daily frequencies): almost none (0), 1–3 times/month (0.1), 1–2 times/week (0.2), 3–4 times/week (0.5), 5–6 times/week (0.8), once/day (1), twice/day (2), and ≥3 times/day (3); the mean portion size was assigned to each food based on a survey of the Japanese participants using 1-day weighed diet records.26 The total energy and macronutrient (carbohydrate, fat, and protein) intake were calculated using a program developed by the Department of Public Health, Nagoya City University School of Medicine, based on the standard tables of food consumption in Japan.22 In the program, the energy from alcohol consumption was included in the total energy intake calculated. A validation study was conducted by comparing the total energy intake and nutrients estimated using the FFQ and 3-day weighed diet records.23 The correlation coefficients were 0.49 (total energy intake), 0.86 (carbohydrate), 0.62 (fat), and 0.50 (protein). The reproducibility correlation coefficient of 1-year intervals in this FFQ ranged from 0.73 to 0.84.
Statistical analysis
In the cross-sectional study, participants were stratified into quartiles (Q1–Q4) based on the macronutrient (carbohydrate, fat, and protein) intake percentage of the total energy intake. We used analysis of variance (for continuous variables) and the χ2 test (for categorical variables) to compare participant characteristics according to the macronutrient quartiles. To examine the overall effects of PA (METs×hour/day) or macronutrient intake (% of total energy intake) on HbA1c (%) and to analyze the effect of interactions between PA and macronutrient intake on HbA1c, we conducted multiple regression analyses using the general linear model. Moreover, we examined the associations between PA and HbA1c in stratified analyses by quartiles of macronutrient intake. Model 1 was adjusted for age (years), sex, study area, total energy intake (kcal/day), alcohol consumption (ethanol g/day), smoking amount (cigarettes/day), and medication for hypertension (yes/no) or hypercholesterolemia (yes/no). Model 2 was further adjusted for BMI (kg/m2).
In the longitudinal study, we examined whether the change in PA (METs×hour/day) between baseline and follow-up (ie, the value at baseline subtracted from the value at follow-up) was associated with the corresponding change in HbA1c (%) and whether this association was modified by quartile-stratified macronutrient intake (carbohydrate, fat, and protein as % energy) at baseline using multiple linear regression analyses. Stratified analyses by quartiles of macronutrient intake at baseline were also performed. Model 1 was adjusted for age at baseline (years), sex, changes (ie, the value at baseline subtracted from the value at follow-up) in each of total energy intake (kcal/day), macronutrient intake (kcal/day), alcohol consumption (ethanol g/day) and smoking amount (cigarettes/day), and medication for each of diabetes (yes/no at follow-up), hypertension (no/no, no/yes, yes/no, and yes/yes at baseline/follow-up) and hypercholesterolemia (categorized as identical to hypertension). Model 2 was further adjusted for BMI change (kg/m2). All analyses were conducted using the SAS statistical software package (V.9.4 for Windows; SAS Institute).
Results
Study participant characteristics are summarized in table 1. The comparison of characteristics according to macronutrient quartiles (carbohydrate, fat, and protein) showed that HbA1c level was not significantly different between protein quartiles (p=0.330). Other variables were significantly different between all macronutrient quartiles (p<0.001). Compared with participants with low-carbohydrate intake, those with high-carbohydrate intake had higher total energy intake and lower fat intake. Moreover, they had higher values for age, HbA1c level, and PA. Compared with participants with low-fat intake, those with high-fat intake had lower total energy intake and lower carbohydrate intake. They also had lower values for age, HbA1c level, and PA.
Characteristics of study participants by lowest quartile (Q) and highest macronutrient (carbohydrate, fat, and protein) intake
Regarding the overall effects of PA or dietary intake on HbA1c level, we observed a weak inverse association between PA and HbA1c level and a significant positive association between total energy or carbohydrate intake and HbA1c level (table 2). No significant associations were observed between fat or protein intake.
Effect of PA, total energy intake, and macronutrient (carbohydrate, fat, and protein) intake on HbA1c
Table 3 shows the associations between PA and HbA1c level according to the quartiles of macronutrient intake. A tendency of interaction between PA and carbohydrate intake on HbA1c level was observed after adjusting for age, sex, study area, total energy intake, alcohol consumption, smoking, and medication for hypertension or hypercholesterolemia. (Pinteraction=0.054) (table 3, Carbohydrate, Model 1). Stratified analyses revealed that the associations between PA and HbA1c level did not reach statistical significance in participants with lower carbohydrate intake (Ptrend=0.636 and 0.917 in Q1 and Q2, respectively), whereas the inverse associations were significant in participants with higher carbohydrate intake (Ptrend=0.021 and 0.005 in Q3 and Q4, respectively). Further adjustment for BMI slightly attenuated the above interaction (Pinteraction=0.098) (table 3, Carbohydrate, Model 2). Similar to the result in model 1, the stratified analyses of model 2 revealed that the associations between PA and HbA1c level were not significant in participants with lower carbohydrate intake (Ptrend=0.679 and 0.786 in Q1 and Q2, respectively). Conversely, the inverse associations were significant in participants with higher carbohydrate intake (Ptrend=0.025 in both Q3 and Q4). There was a significant interaction between PA and fat intake on HbA1c level in model 1 (Pinteraction=0.006) (table 3, Fat, Model 1). Stratified analyses showed that the inverse association between PA and HbA1c level was significant in participants with lower fat intake (Ptrend<0.001 in Q1). However, the associations did not reach statistical significance in participants with higher fat intake (Ptrend=0.639, 0.849, and 0.741 in Q2, Q3, and Q4, respectively). Further adjustment for BMI slightly attenuated the above interaction (Pinteraction=0.068) (table 3, Fat, Model 2). Stratified analyses generated results with the same pattern as in model 1. Moreover, there was no significant interaction between PA and protein intake on HbA1c level (table 3, Protein).
Associations between PA and HbA1c level by quartiles (Q) of macronutrient (carbohydrate, fat, and protein) intake
In the longitudinal study, there was a significant interaction between PA change and carbohydrate intake at baseline on the change of HbA1c level in model 1 (Pinteraction=0.026) (table 4, Carbohydrate, Model 1). Stratified analyses revealed that the association between PA change and HbA1c change was not significant in participants with lower carbohydrate intake (Ptrend=0.717 and 0.273 in Q1 and Q2, respectively), whereas a significant inverse association was observed in participants with higher carbohydrate intake (Ptrend=0.037 and 0.007 in Q3 and Q4, respectively). Further adjustment for BMI slightly attenuated the above interaction (Pinteraction=0.079) (table 4, Carbohydrate, Model 2). Although the interaction between PA change and fat intake on the change of HbA1c level was not statistically significant (Pinteraction=0.257) (table 4, Fat), stratified analyses showed a significant inverse association between PA change and HbA1c change in participants with lower fat intake (Ptrend=0.014 in Q1). However, no significant association was observed in participants with higher fat intake (Ptrend=0.235, 0.616, and 0.621 in Q2, Q3, and Q4, respectively). No significant interaction was noted between PA change and protein intake on the change of HbA1c level (table 4, Protein).
Associations between objectively measured PA change and HbA1c change by quartiles (Q) of macronutrient (carbohydrate, fat, and protein) intake at baseline
Conclusions
This large-scale study revealed the effect of interactions between PA and carbohydrate or fat intake on HbA1c level in the general population. Our finding suggests that the effect of PA on HbA1c levels is modified by the intake of macronutrient composition. The inverse associations between PA and HbA1c level were more evident in participants with higher carbohydrate or lower fat intake than in participants with lower carbohydrate or higher fat intake. Moreover, these associations and interactions were reproduced in the longitudinal study.
Regarding diet, our findings revealed the inverse associations between carbohydrate and fat intake (online supplemental table 1), which appeared as a complementary relationship. Participants with higher carbohydrate intake had lower fat intake, and participants with lower carbohydrate intake had higher fat intake. In participants with high-carbohydrate or low-fat intake, the associations between higher PA and lower HbA1c levels were more evident than those in participants with low-carbohydrate or high-fat intake. Analyses with fasting blood glucose levels as the outcome also showed similar associations to HbA1c (online supplemental table 2) (n=30 416). A previous short-term intervention study demonstrated that low-carbohydrate/high-fat diet decreased the beneficial effects of exercise intervention on blood glucose levels,15 which is consistent with our results.
Supplemental material
In the present study, the interactions between PA and macronutrient intake on HbA1c level were observed after adjusting for total energy intake. This indicates that the effect of PA on HbA1c levels is modified according to the intake of macronutrient composition independent of total energy intake. Dietary energy intake is based on energy-producing nutrients (carbohydrate, fat, and protein). Evidence to determine an appropriate composition without the effects of total energy intake is limited.27 28 Some intervention studies have limited specific nutrients, such as carbohydrate and fat,29 but the groups of limited nutrients have decrease total energy intake at the same time; therefore, it is unable to decide as the effect of specific nutrient limitation alone. The present study demonstrated that the effect of PA on HbA1c level is modified by the macronutrient (carbohydrate or fat) intake after adjusting for total energy intake.
Although further adjustment for BMI slightly attenuated the above interaction in the present analyses, similar associations were observed between PA and HbA1c level in the stratified analyses. This result suggests that the effect of PA is modified by the intake of macronutrient composition, even without obesity-mediated effects. Although a strong association was demonstrated between obesity and type 2 diabetes,30 studies also show that the association differs according to ethnicity,31 and several Asians are known to have type 2 diabetes without obesity compared with other ethnic groups.32 33 Studies have also suggested that Asians have low storage capacity of subcutaneous fat,34 which increases free fatty acid levels and induces insulin resistance.35 Therefore, the effects of macronutrient composition were direct without obesity, and the BMI-mediated effect observed in the present analysis is considered small.
Although the exact mechanisms underlying the interaction between PA and macronutrient intake remain unclear, some mechanisms can explain the findings of the present study. PA improves insulin sensitivity and increases glucose uptake into organs such as skeletal muscle.36–38 Regarding dietary macronutrients, it has been reported that high-fat diet reduces skeletal muscle glucose uptake39 and insulin secretion.40 High-fat intake might weaken the beneficial effect of PA on glucose uptake, due to which the association between PA and HbA1c level was not significant in our study participants with low-carbohydrate or high-fat intake. It has also been reported that a typical high-fat western diet worsened insulin resistance, whereas the traditional high-carbohydrate, low-fat Asian diet improved insulin sensitivity.41 In the present study, it is possible that insulin sensitivity was relatively high in participants with high-carbohydrate or low-fat intake, and increased PA might have further promoted glucose uptake compared with that in participants with low-carbohydrate or high-fat intake. Consequently, the inverse associations between PA and HbA1c level were more evident in participants with high-carbohydrate or low-fat intake than in participants with low-carbohydrate or high-fat intake.
Lifestyle improvements in terms of diet and PA are effective in preventing type 2 diabetes.6–10 The American Diabetes Association and JDS guidelines recommend diet and PA modifications as the first step in diabetes care.27 28 However, the guidelines indicate that there is no ideal dietary composition of carbohydrate, fat, and protein and that individualized instructions are required. The present study findings suggest that increasing PA is particularly effective in reducing HbA1c levels among participants with high-carbohydrate intake (or low-fat intake) similar to that in Japanese whose staple food is rice. Conversely, among individuals who have difficulty in increasing PA for some reasons, decreasing carbohydrate intake may be effective in controlling HbA1c levels (online supplemental table 3). The adjusted means of HbA1c in the joint analysis of PA and macronutrient intake also support the above-mentioned concept (online supplemental table 4).
Our study has several limitations. First, we used self-reported data derived from questionnaires to evaluate diet, which might not be as accurate as objectively measured values. Second, using the total PA, including daily living and leisure-time activity, the effects of differences in activity strength and frequency were not examined. Finally, our study targeted the general population; hence, the results are not applicable to people with diabetes (online supplemental table 5) (n=3366). More detailed examinations, such as evaluating the intensity and frequency of PA and the source and quality of foods, might help in developing more effective preventive and therapeutic approaches.
In conclusion, this study demonstrates the effect of interaction between PA and carbohydrate and fat intake on HbA1c level in the general population. The findings of the present study suggest that the effect of PA on HbA1c levels or blood glucose is modified by the intake of macronutrient composition. The interaction between PA and macronutrient intake probably plays an important role in the control of glucose metabolism. The control of HbA1c could become more effective and sustainable by implementing an optimized diet and PA according to possible interactions as observed in this study.
Data availability statement
Data are available upon reasonable request. Details can be found on the J-MICC Study website (http://www.jmicc.com/).
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the ethics committee of Nagoya University Graduate School of Medicine (approval number: 253) and all other study centers. Participants gave written informed consent to participate in the study before taking part.
Acknowledgments
The authors thank former principal investigators (Drs Nobuyuki Hamajima and Hideo Tanaka) and all the participants and staff who cooperated in the J-MICC Study. They also thank the following researchers for providing them with a Food Frequency Questionnaire and program to calculate nutrient intake: Shinkan Tokudome at the National Institute of Health and Nutrition (formerly Nagoya City University), Chiho Goto at Nagoya Bunri University, Nahomi Imaeda at Nagoya Women’s University, Yuko Tokudome at Nagoya University of Arts and Sciences, Masato Ikeda at University of Occupational and Environmental Health, and Shinzo Maki at Aichi Prefectural Dietetic Association.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Collaborators A list of study group investigators (J-MICC Study Group): Kenji Wakai, Kenji Takeuchi, Haruo Mikami, Hiroki Nagase, Hiroto Narimatsu, Kiyonori Kuriki, Keitaro Matsuo, Sadao Suzuki, Asahi Hishida, Yoshikuni Kita, Katsuyuki Miura, Ritei Uehara, Kokichi Arisawa, Hiroaki Ikezaki, Keitaro Tanaka, Toshiro Takezaki.
Contributors TF, YNi, MH, KTan, and KW designed the study. TF, YNi, MH, CS, KKo, CI, and KTan interpreted the data and contributed to the discussion. YNi, MH, CS, YH, KTan, RN, HIk, AH, TT, YK, YT, KM, HIt, HM, MK, RI, KS, SS, HN-S, EO, DM, KKu, YNa, AK, KA, SK-K, KTak, and KW collected the data. TF and MH conducted the data analysis. TF and YNi drafted and edited the manuscript. All authors read and approved the final manuscript. TF is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding This study was supported by Grants-in-Aid for Scientific Research for Priority Areas of Cancer (No. 17015018) and Innovative Areas (No. 221S0001) and by JSPS KAKENHI Grants (Nos. 16H06277 (CoBiA) and 21K06645) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.