Article Text
Abstract
Introduction Dapagliflozin, a sodium-glucose transporter inhibitor, effectively reduces blood glucose and is indicated for individuals with kidney diseases and cardiovascular disorders. In this study, we further expand the therapeutic benefit of dapagliflozin in the neural and vascular retina, with the potential to effectively manage diabetic retinopathy (DR), the most common complication of diabetes.
Research design and methods Db/db mice, an animal model of type 2 diabetes, were treated with dapagliflozin orally, and the electroretinogram (ERG) response and acellular capillary numbers were assessed. Messenger RNA levels of inflammatory cytokines were studied using real-time quantitative (q)PCR. We assessed endothelial cell migration in a scratch wound assay and retinal glucose uptake using human retinal endothelial cells.
Results The dapagliflozin treatment improved the ERG b-wave amplitude and decreased acellular capillary numbers. The scratch wound assay demonstrated a reduction in wound closure after dapagliflozin treatment. Retinal glucose uptake reduced after dapagliflozin treatment compared with the respective controls.
Conclusions Our studies suggest that dapagliflozin treatment effectively corrects neural and vascular dysfunction of the retina in diabetes. This effect is mediated by a decrease in inflammation and improved glycemic control. In addition, dapagliflozin exhibits decreased wound healing and glucose uptake, which could benefit the retina. Thus, dapagliflozin could be helpful in the management of DR, with multimodal therapeutic effects.
- Diabetic Retinopathy
- Retina
- Diabetes Mellitus, Type 2
- Diabetes Complications
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Diabetic retinopathy (DR) is a sight-threatening and most common complication of diabetes.
Hyperglycemia is central to the pathogenesis of DR.
Dapagliflozin, a sodium-glucose transporter-2 inhibitor, promotes glucose excretion, but its role in the treatment of DR is unknown.
WHAT THIS STUDY ADDS
Dapagliflozin was effective in correcting electroretinogram abnormalities.
Dapagliflozin treatment reduced acellular capillaries and inflammation in the retina.
Dapagliflozin treatment of human retinal endothelial cells resulted in a decrease in glucose uptake and wound healing.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
Dapagliflozin treatment could be used clinically in the future to manage neural and vascular dysfunction in diabetes.
Introduction
Diabetic retinopathy (DR) is a microvascular complication of diabetes. DR is the most significant contributor to blindness in the working-age population and its prevalence is increasing. It is estimated that at least 50% of patients with type 1 or type 2 diabetes will develop DR throughout their lives.1 DR is characterized by ischemia in the early phase of the disease, which further triggers the release of a variety of cytokines, including vascular endothelial growth factor (VEGF), resulting in the formation of new blood vessels from the parent blood vessels. While DR is mainly a disease of vascular dysfunction, changes in the neural retina precede vascular changes. DR exhibits changes in retinal b-wave and oscillatory potential (OP) amplitudes throughout the condition.2 Therefore, evaluation of the functional properties of the retina using retinal electroretinogram (ERG), electro-oculography, and visual evoked cortical potentials is vital to diagnosing, understanding, and developing treatment approaches for DR.2
Hyperglycemia is known to be a major factor in the pathogenesis of DR.3 Within the retina, excess intracellular glucose can overwhelm the glycolytic pathway and get shunted to alternative paths, such as the polyol pathway, resulting in oxidative, proinflammatory, proangiogenic, byproducts, and end products.1 4 5 These products contribute to the microvascular dysfunction that is the hallmark of DR, leading to ischemia, angiogenesis, and ultimately vision loss. Treatment choices such as pan-retinal photocoagulation work at the expense of the retina and anti-VEGFs are effective only at the later stages of DR and some individuals are non-responsive to these treatments.6 Therefore, there is an ensuing need to develop newer targets and treatments for DR.
Sodium-glucose cotransporter-2 (SGLT2) is expressed throughout the body, most notably within the proximal convoluted tubules of the kidneys, where it is responsible for the reabsorption of 90% of the filtered glucose.7 SGLT2 inhibition has been well studied within the kidney and the heart, and has been found to have nephroprotective7 and cardioprotective effects in patients with type 2 diabetes.8 SGLT2 expression within the retina has also been studied, where it has been found to be expressed around the microvasculature.9 Phlorizin, a dual inhibitor of SGLT1 and SGLT2, has been shown to correct hyperglycemia, and reduce cell death, insulin signaling defects, and retinal gliosis in diabetes.10 Also, animal studies suggest that an SGLT2 inhibitor, ipragliflozin, effectively reduces cataract formation, ERG abnormalities, and irregularities of the outer nuclear layer (ONL).11 Dapagliflozin is another SGLT2 inhibitor indicated for glycemic control in diabetes and treating adults with kidney failure and heart failure with reduced ejection fraction.12 13 Dapagliflozin treatment reduces retinal capillary hyperperfusion and arteriolar remodeling.14 Moreover, modeling studies predict that SGLT2 inhibitors may reduce the incidence of DR by 10%.15 Dapagliflozin has also been shown to improve visual acuity in individuals with diabetic macular edema (DME).16 Additionally, dapagliflozin treatment had a beneficial outcome in retinal arteriolar modeling and retinal capillary flow.14
This study aimed to address the gap in understanding the protective effect of dapagliflozin in DR. Our study shows that dapagliflozin treatment results in better glycemic control, preservation of visual function, and decreased microvascular dysfunction in mice. In addition, we also report that, at the cellular level, dapagliflozin treatment led to a decrease in wound healing and glucose uptake in retinal endothelial cells.
Methods
Animal care
All animal care and experimental conditions followed the Guiding Principles in the Care and Use of Animals (National Institutes of Health) and the Association for Research in Vision and Ophthalmology’s Statement for the Use of Animals in Ophthalmic and Vision Research. Five-week-old male B6.BKS-Leprdb (homozygous-db/db (n=20), heterozygous db/m (n=10); #000697) were purchased from Jackson Laboratory (Bar Harbor, Maine). The db/db mice were treated with a test diet (Research Diets, New Brunswick, New Jersey) containing dapagliflozin (10 mg/kg/day) based on previous reports.17 Another group of db/db mice and db/m animals received a regular diet from 8 to 9 weeks of age. The weight and food consumption of the mice were assessed weekly for 28 weeks, and hemoglobin A1c (HbA1c) was measured at 10 and 29 weeks after starting dapagliflozin treatment. The mice were euthanized between 29 and 31 weeks after the start of dapagliflozin treatment.
Electroretinogram
Both scotopic and photopic measurements were also taken at 12 and 29 weeks before study termination. The mice were anesthetized with ketamine/xylazine mixture, and 1% tropicamide/2.5% phenylephrine was given for pupillary dilation. Gonak (Hypromellose 2.5% Solution; Akorn, Lake Forest, Illinois, USA) was applied and then a gold loop electrode (LKC Technologies, Gaithersburg, Maryland, USA) was placed over the cornea. ERG recordings were performed using an LKC NGIT-100 recording machine (LKC Technologies); a-wave and b-wave amplitude values were obtained using analysis tools from LKC Technologies.
Complete blood count analysis
Blood was collected in EDTA-coated small microcentrifuge tubes (Microvette; Sarstedt AG & Co KG, Germany). Complete blood count (CBC) analysis was performed using an automated Element HT5 Veterinary Hematology Analyzer (Heska Corporation, Loveland, Colorado) on 15 µL of blood. The instrument counts different blood parameters as outlined in online supplemental table 1.
Supplemental material
Acellular capillary analysis
Animals were euthanized and the eyes were enucleated and fixed in 4% paraformaldehyde. A day before trypsin digestion, a small incision was made in front of the eyeball using a fine scalpel blade (#11) to separate the retina. Through this small cut using vannas scissors, the eye was cut behind the ora serrata separating the anterior and posterior portions of the eye. Next, the lens was removed and the posterior cup of the eye was placed in a petri dish containing phosphate-buffered saline (PBS). Finally, the retina was separated by carefully dissecting the scleral layer. The isolated retina was placed in 50 mL water for unfixing overnight. The individual retina was incubated in 3% trypsin at 37°C for 2 hours the next day. Trypsin digested retina was placed in a petri plate and the internal limiting membrane was gently separated from the peripheral retina with fine forceps. Then using vannas scissors, the internal limiting membrane was isolated from an optic nerve. Subsequently, the neural retina was removed and the isolated retinal vasculature was stained with periodic acid and Schiff’s base to assess acellular capillary numbers.
Inflammatory marker studies
RNA was extracted from the retinal samples using Trizol (ThermoFisher Scientific) and 1 μg of RNA was used to prepare cDNA using SuperScript Vilo Kit. A real-time quantitative reverse transcription (qRT)-PCR was performed using gene-specific primers for the following markers: adiponectin (Mm00456425_m1), interleukin 1 beta (IL-1β) (Mm00434228_m1), tumor necrosis factor-alpha (TNF-α) (Mm00443258), and ACE2 (Mm01159003_m1).
Cell culture
Human retinal endothelial cells (HREC; Cell Systems, Kirkland, Washington) were grown in complete endothelial base media (EBM-2 and EGM-2 MV Kit; Lonza, Walkersville, Maryland, USA). Cells from passages 3–7 were used in the experiments. To obtain optimum dosing range, viability was determined using Alamar blue assay (ThermoFisher Scientific).
Wound healing and migration assay
HRECs were plated onto 24-well tissue culture plates and treated with dapagliflozin (0.1, 1, 10, 50, and 100 µM) and ethanol (EtOH) controls (0.05%–0.00005%). A P200 pipette tip was used to create a scratch in each well and wound healing. Images were taken using AMG EVOS FL Digital Inverted Imaging System at 8 hours after adding dapagliflozin. The width and area of the wound on the images were measured using ImageJ. The percentage of wound healing were determined using the following formula and as described18:  , where At=0h is the area of wound closure immediately after the scratch wound and At=Δh is the area of the wound measured at h hours after initial scratch.
, where At=0h is the area of wound closure immediately after the scratch wound and At=Δh is the area of the wound measured at h hours after initial scratch.
Glucose uptake assay
Glucose uptake was studied with HRECs. The cells were grown to 70% confluency, then plated at 104 cells/well in a 96-well plate overnight in complete EBM-2 media. Following this, they were treated with dapagliflozin (0.1, 1, 10, 50, and 100 µM) or EtOH controls (0.05%–0.00005%) and simultaneously glucose-starved in Krebs Ringer phosphate HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer (136 mM NaCl, 20 mM HEPES, 5 mM K2HPO4, 4.7 mM KCl, 1 mM MgSO4, and 1 mM CaCl2, pH 7.4) for 90 min at 37°C. Following this, the cells were incubated with 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) for 30 min, then washed with PBS twice. The plate was then analyzed at an excitation/emission maxima of 485/535 nm to determine glucose uptake based on the intracellular fluorescence of the 2-NBDG using a microplate reader (Synergy H1; BioTek, Winooski, Vermont, USA).
Statistics
All data were expressed as mean±SD and statistical analysis was performed using GraphPad Prism V.9.0.0 for Windows (San Diego, California; www.graphpad.com). Except for real-time qRT-PCR, all data were analyzed using either one-way or two-way analysis of variance, followed by Fisher’s least square design (LSD) test. Real-time qRT-PCR data were analyzed using IBM SPSS Statistics V.27 (www.ibm.com/products/spss-statistics). Statistical significance for real-time qRT-PCR was tested using linear mixed model estimated marginal (EM) means, followed by a comparison of a particular group with LSD. Data were considered statistically significant when the p value was less than 0.05.
Results
Dapagliflozin treatment resulted in improved glycemic control
The db/db mice were found to have higher weights and food intake throughout the study than the db/m mice; the treated mice showed no significant change in either (online supplemental figure 1A,B). Despite this, dapagliflozin treatment reduced HbA1c levels significantly compared with the untreated db/db mice at 10 weeks (p<0.01; online supplemental figure 1C). Just before study termination, the treated mice still had lower HbA1c levels, but at this point the difference was no longer significant (online supplemental figure 1D).
Supplemental material
ERG changes in mice treated with dapagliflozin. Line graph showing different parameters of ERG under scotopic conditions: (A) b-wave amplitude, (B) b-wave implicit time, (C) a-wave amplitude, (D) a-wave implicit time, (E) oscillatory potential and photopic conditions, (F) b-wave amplitude, (G) b-wave implicit time, (H) a-wave amplitude, (I) a-wave implicit time, and (J) oscillatory potential. n=8 db/m; n=5 db/db; n=6 db/db+Dapa. Data were analyzed using two-way ANOVA, followed by Fisher’s LSD test. ANOVA, analysis of variance; Dapa, dapagliflozin; ERG, electroretinogram; LSD, least square design.
Improved b-wave ERG
Since previous studies showed protection in ERG response after an SGLT2 inhibitor treatment and changes in neural retina often precede vascular dysfunction observed in DR,11 we performed an ERG at 12 and 29 weeks after starting dapagliflozin treatment. The a-wave corresponds to the response of the photoreceptor cells and the b-wave corresponds to the bipolar neurons. Out of all parameters tested, at 12 weeks, there was only a significant change in photopic a-wave amplitude and implicit time after dapagliflozin treatment (online supplemental figure 2H). Diabetes resulted in a significant increase in the a-wave amplitude (p<0.05), which decreased significantly after dapagliflozin treatment (p<0.01). Other parameters of ERG are described in online supplemental figure 2A-J.
Supplemental material
Decrease in acellular capillary number after dapagliflozin treatment. (A) Representative images of trypsin digested retinas showing changes in acellular capillary numbers (black arrows). (B) Bar chart showing enumeration of acellular capillaries. n=9, db/m; n=8 db/db; n=5 db/db+Dapa. One-way ANOVA, followed by Fisher’s LSD test. *P<0.05, ***P<0.001. ANOVA, analysis of variance; Dapa, dapagliflozin; LSD, least square design.
At 29 weeks, the b-wave recordings showed a decrease in the b-wave amplitude under both scotopic (figure 1A) and photopic (figure 1F) conditions in the db/db mice compared with the db/m mice. While this difference was significant (p<0.05) for the b-wave amplitude under scotopic conditions (figure 1A), this decrease did not reach statistical significance for photopic conditions. Dapagliflozin treatment corrected the defects in ERG amplitude significantly at both scotopic (p<0.05 and p<0.01; figure 1A) and photopic (p<0.05; figure 1F) conditions. The implicit time of the b-wave for db/db mice was significantly higher (p<0.05; figure 1B) when compared with db/m mice under scotopic conditions. Dapagliflozin treatment corrected the implicit time for both scotopic (figure 1B) and photopic (figure 1G) conditions; however, statistical significance was achieved only under photopic conditions (p<0.05; figure 1G).
There was no change in the a-wave amplitude in diabetic mice under scotopic conditions; however, dapagliflozin treatment significantly improved the a-wave amplitude (figure 1C). The db/db mice under photopic conditions exhibited a significant increase in the a-wave amplitude (p<0.05; figure 1H), and while dapagliflozin treatment did decrease photopic a-wave amplitude this difference was not statistically significant. The implicit time for the a-wave increased under both scotopic (figure 1D) and photopic (figure 1I) conditions, and dapagliflozin treatment indeed corrected these defects. Statistical significance was only achieved under photopic conditions (p<0.01; figure 1I).
OP is known to be decreased in individuals with DR.19 We observed a similar and significant decrease in OP under scotopic conditions (p<0.01; figure 1E) and dapagliflozin treatment helped correct the reduction in OP amplitude. There was also an improvement in OP under photopic conditions (figure 1J).
Decrease in acellular capillaries
An increase in acellular capillary number is a pathological hallmark of DR.20 The db/db retinas showed a significant increase in the number of acellular capillaries compared with db/m retinas (p<0.001; figure 2A,B). Dapagliflozin-treated retinas showed a substantial decrease in the number of acellular capillaries compared with the db/db retinas (p<0.05). No significant difference was observed between the treated retinas and the db/m retinas (figure 2A,B).
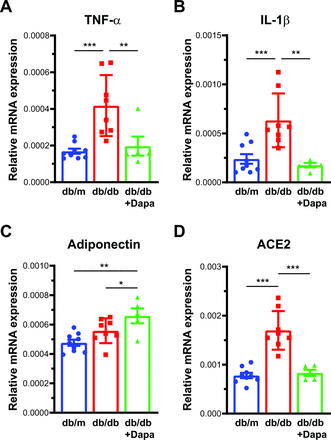
Decrease in IL-1β, TNF-α, ACE2, and adiponectin
Diabetes is known to create an inflammatory milieu, increasing cytokines. To understand whether the beneficial effect of dapagliflozin on retinal ERG and acellular capillary numbers was due to a decrease in inflammation, we studied the messenger RNA (mRNA) levels of inflammatory cytokines. TNF-α and IL-1β, both known to be upregulated in the retina in diabetes and associated with retinopathy,21 were significantly upregulated in the db/db mice and dapagliflozin treatment corrected this increase significantly (figure 3A,B).
mRNA expression in the retina. Bar chart showing mRNA levels on (A) TNF-α, (B) IL-1β, (C) adiponectin, and (D) ACE2, after dapagliflozin treatment. n=9, db/m; n=8, db/db; n=5 db/db+Dapa. The mRNA expression was determined in triplicate for each mouse and represented as an average data point for an individual mouse. Raw data were analyzed using linear mixed model EM means, followed by comparison of a particular group with LSD. *P<0.05, **P<0.01, ***P<0.001. Dapa, dapagliflozin; EM, estimated marginal; IL-1β, interleukin 1 beta; LSD, least square design; mRNA, messenger RNA, TNF-α, tumor necrosis factor-alpha.
Adiponectin is a known marker of insulin sensitivity, and low adiponectin levels were associated with increased angiogenesis. Therefore, we studied the mRNA levels of adiponectin mRNA.22 Dapagliflozin treatment increased the expression levels of adiponectin significantly in the db/db retinas compared with the db/m (p<0.01) and the untreated db/db (p<0.05) retinas (figure 3C). ACE2 has been known to be increased in retinopathy in human retinal tissue,23 and dapagliflozin treatment could be protective. We reasoned to test this hypothesis, and there was a significant (p<0.001) decrease in ACE2 mRNA after treatment with dapagliflozin (figure 3D).
Dapagliflozin treatment results in a decrease in wound healing and glucose uptake in HRECs
To further gain mechanistic insights into the protective action of dapagliflozin, we decided to conduct in vitro studies using HREC. Before beginning further studies, the viability of HRECs was determined using an Alamar blue assay. The HRECs treated with dapagliflozin HRECs were viable at a variety of dapagliflozin concentrations (0.0001–100 000 nM). More than 98% of HRECs were viable up to 10 µM concentration of dapagliflozin; however, the viability decreased to 94% and 65% at 50 µM and 100 µM of dapagliflozin treatment (online supplemental figure 3).
Supplemental material
In the series of studies to understand the cellular mechanism of action of dapagliflozin, we first performed a scratch wound assay in HRECs. Figure 4A shows representative images of the resulting scratch wounds 8 hours after injury. As seen in figure 4B, dapagliflozin treatment resulted in significantly slower wound healing at 50 µM (p<0.05) and 100 µM (p<0.001).
Wound healing after dapagliflozin treatment. (A) Representative photomicrographs showing scratch wound assay after dapagliflozin treatment and respective alcohol controls. (B) Bar chart showing quantification of per cent wound healing. Data were analyzed using one-way ANOVA, followed by Fisher’s LSD test. *P<0.05, ***P<0.001. n=32, medium only; n=12, control and treatment. ANOVA, analysis of variance; CTRL, vehicle control; Dapa, dapagliflozin; LSD, least square design.
Since we observed an overall improvement in glycemic control after dapagliflozin treatment as suggested by a decrease in HbA1c, we further wanted to study whether there is any effect on glucose uptake at the cellular level. Dapagliflozin treatment induced a decrease in glucose uptake at all treatment levels compared with the respective EtOH controls (figure 5). There was significant decrease at 1 µM (p<0.001) and 10 µM (p<0.05).
Decrease in glucose uptake after dapagliflozin treatment. Bar chart showing 2-NBDG uptake in HRECs treated with dapagliflozin at different concentrations and respective controls. The results were tested for significance using one-way ANOVA, followed by Fisher’s LSD test. *P<0.05, ***P<0.001. n=14. 2-NBDG, 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose; ANOVA, analysis of variance; CTRL, vehicle control; Dapa, dapagliflozin; HRECs, human retinal endothelial cells; LSD, least square design.
Dapagliflozin treatment improves hematocrit and mean corpuscular volume
Since previous studies demonstrated that dapagliflozin treatment improves hematocrit and that lower hematocrit levels are reported in individuals with DME,24 25 we performed a CBC analysis of blood obtained from the db/db mice. While there was no change in the number of types of blood cell numbers, there was a significant increase in hematocrit (Hct) and mean corpuscular volume (MCV) in mice treated with dapagliflozin (online supplemental table 1).
Discussion
Dapagliflozin is an effective antidiabetic, and due to its multimodal benefits it has been implicated in treating various conditions, including chronic kidney disease, heart failure, and non-alcoholic fatty liver diseases.26 In addition, an SGLT2 inhibitor, tofogliflozin, has previously been shown to be effective in reducing glial fibrillar acidic protein and VEGF activation in db/db mice. Empagliflozin reduced preclinical DR, highlighting the potential benefit of SGLT2 inhibition in DR.27–29 Our studies further add to the evolving role of SGLT2 inhibitors by showing the protective effect of dapagliflozin on retinal vascular and neural dysfunction. The protective effect was mainly mediated by improvement in systemic glycemic control, decrease in inflammation, decrease in wound healing response and glucose uptake, and improvement in hematocrit levels, which together helped to induce a protective effect on the neural and vascular retina.
Our findings demonstrate a protective effect of dapagliflozin on the neural retina and vascular network, as indicated by improvement in ERG response and decrease in acellular capillary numbers. During the early stages of DR, that is, non-proliferative DR, there is a decrease in ERG b-wave amplitude and OP. With proliferative DR, these abnormalities could be even more severe;2 30 therefore, protection of ERG response could be significant in the case of DR. We report improvement in ERG b-wave amplitude under both scotopic and photopic conditions. Our studies agree with previous reports that suggest protection from ERG response, where ipragliflozin treatment resulted in a decrease in the OP response of diabetic animals.11 Phlorizin treatment also had a similar protective effect on ERG response.31 In previous studies, protection in ERG response was mainly attributed to the correction of the ONL.11 DR can decrease phototransduction and visual perception within the bipolar cells in animal studies,32 and our findings support this with a reduction in the b-wave amplitude in untreated diabetic animals. Our studies suggest an involvement of ON bipolar cells and photopic cone response in the protective effect of observed dapagliflozin treatment. However, further studies are required to clarify the significance of retinal cellular involvement.
Along with changes in ERG, we also observe protection from an increase in acellular capillary numbers with dapagliflozin treatment. Acellular capillaries consist mainly of basement membrane with little to no endothelial cells, and as a result they are notoriously leaky vessels, contributing greatly to retinal ischemia,33 and are a hallmark finding in patients with DR.34 Seeing the decrease indicates that dapagliflozin treatment prevents the microvascular dysfunction underlying the formation of acellular capillaries and slows down the progression of DR in the treated mice. One of the defining mechanisms of preventing an increase in acellular capillaries is improvement in systemic glycemic control, as indicated by a decrease in glycated hemoglobin at both time points. Our studies on glucose uptake assay further suggest that a decrease in local glucose uptake could also play a role in protecting from acellular capillary formation. Wakisaka and Nagao9 previously reported a reduction in glucose uptake in retinal pericytes. They propose a potential mechanism of decreasing glucose uptake in retinal pericytes through Na+–Ca2+ exchanger that modulates intracellular Ca2+ concentration. This exchanger works by promoting glucose and sodium influx when the extracellular glucose concentration is high and blockage of SGLT2 indirectly inhibits intracellular glucose uptake.
Previous studies suggest that SGLT2 inhibitors can work directly or indirectly on a variety of tissues, such as cardiac, renal, endothelial, and immune cells, to reduce inflammation.35 Inflammation is central to the pathogenesis of DR, and both TNF-α and IL-1β have been implicated in the development of DR.21 36 37 In our studies, dapagliflozin-treated mice exhibited a decrease in both these cytokines. These results agree with previous studies where dapagliflozin treatment reduced serum TNF-α in clinical samples and IL-1β in animal studies.38 39 The mechanism by which dapagliflozin exercises its anti-inflammatory activity is multifactorial and not mutually exclusive. First, it is known that dapagliflozin treatment reduces body weight and postprandial blood glucose.35 While we did not observe any evidence of weight reduction after dapagliflozin treatment, there was a decrease in glycated hemoglobin during treatment. Second, dapagliflozin treatment is known to decrease serum insulin levels, and hyperinsulinemia is a known driver of adipose tissue inflammation and proinflammatory macrophages. While we did not study insulin levels, the decrease in adiponectin levels signals the involvement of similar mechanisms; indeed higher levels of adiponectin are associated with greater insulin sensitivity,40 while low adiponectin levels are associated with increased angiogenesis and development of DR. A meta-analysis study involving 10 clinical trials demonstrating a decrease in adiponectin levels due to SGLT2 inhibitors further supports our observation that promoting insulin sensitivity could also be a potential mechanism of decreased inflammation.41 In addition, dapagliflozin reduced inflammation by multiple other mechanisms, such as suppression in advanced glycation end (AGE) product and receptor for AGE axis. Activation of adenosine monophosphate-activated protein kinase could also play a key role in inducing beneficial effects of dapagliflozin treatment.35
It is noteworthy that a smaller concentration of 1 µM was sufficient to decrease glucose uptake in HRECs; however, a higher dose was necessary to exhibit wound healing response, suggesting a concentration-dependent action of dapagliflozin. Previous studies show that dapagliflozin exhibits a dose-dependent action. At a lower concentration of 0.1–20 µM, there was no effect on cell proliferation; however, the inhibitory effect was observed at a higher concentration of 30–50 µM. Dapagliflozin’s protective antioxidant action could partly explain this effect at lower concentrations (0.1–5 µM).42 Additionally, dapagliflozin (1 µM) treatment of HRECs did not affect glucose uptake; however, there was a decrease in high glucose-induced arachidonic acid increase. This action was mainly mediated through a decrease in phosphorylation of extracellular-signal-regulated kinase (ERK)1/2 (mitogen-activated protein kinase) and cytosolic phospholipase A2.43 This suggests that dapagliflozin possesses dose-dependent and tissue-dependent effects. Our study highlights that dapagliflozin could be beneficial in the later stages of DR, such as proliferative DR, where nascent blood vessels progress from the parent blood vessels. In a similar study, canagliflozin, another SGLT2 inhibitor, has been shown to reduce the migration and proliferation of human umbilical endothelial cells under similar concentrations.44
Additionally, notable findings of our study include a decrease in ACE2 mRNA by dapagliflozin treatment. ACE2 is known to be upregulated in the retinal vascular tissue of individuals with DR.23 ACE2 is a well-known entry receptor for the SARS-CoV-2 virus, with implications for comorbidities of COVID-19; however, a recent clinical trial in hospitalized patients with cardiometabolic risk factors failed to show a significant reduction in organ dysfunction, clinical recovery, or death.45 46 ACE2 is known to be increased in the renal tubule of db/db mice;47 however, some reports suggest that ACE2 could be protective in DR due to correction of bone marrow dysfunction.48 49 These reports and our study certainly pinpoint the critical role of ACE2 in retinal pathology and pave the way for future studies in the context of DR and SARS-CoV-2.50
Another exciting aspect of our study is improving the hematocrit and MCV after treatment with dapagliflozin, in line with published reports.24 It is known that lower hemoglobin levels are associated with retinal ischemia and severity of DR, suggesting that low oxygen-carrying capacity is involved in the pathogenesis of DR; however, a mechanism could be complex and warrants further research towards multimodal action of dapagliflozin.25
We used db/db mice as an animal model to test our hypothesis. In a recent study, dapagliflozin’s protective effect was tested in the streptozotocin-induced diabetes mouse model where dapagliflozin helped to improve retinal thickness; this protective effect was mediated through antiapoptotic action.43 Our ERG studies point to measuring retinal thickness using assays such as spectral-domain optical coherence tomography or similar to the above study; however, the current study design did not permit this and testing for retinal apoptosis, which we consider as a limitation of the study. Additionally, it would also be interesting to study dapagliflozin in animal models such as high-fat-diet-induced diabetes and assess retinopathy for blood–retinal barrier integrity or leukostasis. While these were not studied with the present study design, our study indeed paves the way for such studies in the future.
Conclusion
In conclusion, our studies demonstrate that dapagliflozin effectively reduces neural and vascular dysfunction observed in the retina. Furthermore, the protective effect of dapagliflozin is mediated through direct and indirect actions via a combination of mechanisms such as better glycemic control, decrease in inflammation, cell migration, and cellular glucose uptake. Thus, dapagliflozin is promising in treating neural and vascular dysfunction of the retina in diabetes; however, further studies are needed to see how well these findings translate to humans.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
The Institutional Animal Care and Use Committee (IACUC) of Indiana University School of Medicine approved the study (protocol number #19064).
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Presented at Part of this study was discussed at an eye conference. Bhatwadekar AD, Mathew D, Dhami H, Bello E, Luo Q. Dapagliflozin treatment protects from diabetic retinopathy. Investigative Ophthalmology & Visual Science, June 2021, Vol. 62, 1161.
Contributors QL, SPL, EB, HD, DM and ADB performed the experiments and analyzed the data. QL helped supervise the project. SPL and ADB wrote the manuscript. ADB devised the project and the main conceptual idea, and was in charge of overall direction and planning. All authors discussed the results and contributed to the final manuscript. ADB is the guarantor of the study and accepts responsibility for the manuscript’s overall integrity, including study conduct, reporting of the results, data handling, and ethics.
Funding This project was directly supported by a Pilot and Feasibility Award within the CDMD NIH/NIDDK (grant number P30 DK097512). In addition, the research in the laboratory of ADB is supported by funding from the National Institutes of Health (NIH)-National Eye Institute (NEI) (grants R01EY027779 and R01EY032080) and a challenge grant from the Research to Prevent Blindness to the Department of Ophthalmology.
Competing interests ADB is an ad hoc staff pharmacist at CVS Health/Aetna. The content of this study does not reflect those of CVS Health/Aetna. QL, SPL, EB, HD, and DM do not have any conflicts of interest with the study.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.