Article Text
Abstract
Objective Patients with diabetes have an increased risk for urolithiasis, but the associated risk factors remain an active area of research. We investigated whether frailty influenced the probability of patients with diabetes developing urolithiasis.
Research design and methods Using data from the Longitudinal Cohort of Diabetic Patients from 2004 to 2010, we identified those without and with frailty based on a validated, modified FRAIL scale. Patients were followed until they developed urolithiasis, and we used Kaplan-Meier and Cox proportional hazard regression analyses to examine the relationship between frailty, its severity, and the risk of urolithiasis, accounting for demographic profiles, comorbidities, frailty status changes over follow-up, and medications, with risk competition by mortality.
Results Among 525 368 patients with diabetes, 64.4% were not frail, while 28.5%, 6.6%, and 0.6% had 1, 2, and ≥3 FRAIL items at baseline. After 4.2 years of follow-up, 13.4% experienced incident urolithiasis. Cox proportional hazard regression analysis showed that patients with diabetes having at least one FRAIL criterion exhibited a significantly higher risk for urolithiasis compared with non-frail patients (for 1, 2, and ≥3 items, hazard ratio (HR)s: 1.04, 1.23, and 1.46; 95% confidence intervals (CIs) 0.99 to 1.09, 1.12 to 1.35, and 1.12 to 1.91, respectively). This increase in urolithiasis risk remained significant if we restricted analyses to renal stones or recurrent urolithiasis as the study outcomes.
Conclusions Frailty may pose a risk for incident urolithiasis in patients with diabetes. Treating frailty may potentially reduce their risk for urolithiasis.
- chronic kidney disease
- diabetes mellitus
- frail phenotype
- frailty
- renal stone
- urinary stone
- urolithiasis
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Significance of this study
What is already known about this subject?
Patients with diabetes already have a significantly higher risk for developing urolithiasis.
What are the new findings?
Patients with diabetes with physical frailty exhibited a higher risk of incident urolithiasis than those without frailty, with a dose-responsive relationship.
How might these results change the focus of research or clinical practice?
The assessment of frailty can assist in estimating the risk of urolithiasis among patients with diabetes.
There is a probability that interventions directed toward frailty can ameliorate the risk of developing urolithiasis among patients with diabetes.
Background
The proportion of individuals having diabetes mellitus (DM) is increasing worldwide, and the complications associated with DM, including cardiovascular diseases, nephropathy, and retinopathy, are responsible for the majority of the morbidity and mortality among these patients. Apart from these well-known complications, diabetes has recently been reported to be a risk for developing urolithiasis; a population-based study revealed that patients with diabetes had an 18% higher probability of developing urinary tract stones compared with those without diabetes.1 Another study using the National Health and Nutrition Examination Survey (NHANES) similarly showed that DM significantly increased the risk of developing renal stones by 80%–180%, with the risk rising incrementally with worsening glycemic control.2 Urolithiasis poses a particular concern due to its adverse prognostic influences. The presence of urolithiasis necessitates medical or invasive stone management and negatively influences patients’ quality of life. Prior studies further suggested that urolithiasis significantly increased the risk of progressing to end-stage renal disease independent of cardiovascular morbidities and future stroke.3 In light of these findings, it is imperative that we place more emphasis on urolithiasis, an under-recognized complication among patients with DM, regarding its presence and associated risk factors in this population.
The relationship between DM and the risk of urolithiasis has been previously attributed to insulin resistance-related urine acidification, hypercalciuria induced by elevated plasma insulin levels following exaggerated dietary carbohydrate, the coexistence of morbidities (ie, hypertension or hyperuricemia), and dietary preferences (ie, increased dietary sodium).4 5 Although metabolic disorders, including DM, increase the tendency for stone formation, it remains unclear whether there are any specific risk factors that further modulate the risk of urolithiasis in patients with such disorders.
Patients with DM are at a higher risk of exhibiting frailty, a degenerative trait characterized by a higher vulnerability to endogenous or environmental injuries, regardless of their age, likely due to the combinatorial effects of hyperglycemia-induced premature cellular senescence, chronic inflammation, and oxidative stress.6 Accumulating evidence indicates that frailty in patients with diabetes causes adverse outcomes ranging from effects on mortality and functional independence, to musculoskeletal degeneration, an increased risk of cardiovascular events, and renal failure.7 8 None of the existing studies has examined whether frailty may influence the probability of urolithiasis in patients with DM. We hypothesized that frail patients with diabetes might have a significantly higher risk of developing urolithiasis compared with those without frailty. To increase the statistical efficacy, we used a large cohort of patients with DM to investigate this hypothesis.
Methods
Assembly of the study cohort
We selected patients with DM from the Longitudinal Cohort of Diabetes Patients (LCDP), a well-maintained cohort of patients with diabetes derived from an annual random selection of 120 000 patients with at least one diagnosis of diabetes from the National Health Insurance Database in Taiwan, between 2004 and 2010.7 8 This led to an initial cohort size of 840 000 during the study period. We further restricted the diagnostic criterion for DM to a minimum of three outpatient diagnoses of diabetes (International Classification of Disease 9th version—Clinical Modification (ICD-9-CM) code 250.x) or at least one inpatient diagnosis, a validated approach for increasing the specificity of DM diagnosis.7 8 The exclusion criteria consisted of pediatric cases (age≤20 years), those with missing data, with any codes of the outcome prior to the diagnosis of DM, and those with insufficient length of follow-up (at least 1 year after the date of DM diagnosis, no later than December 31, 2010). On identification, we recorded their demographic profile (ie, age and sex), lifestyle factors (smoking, alcoholism, and obesity), comorbidities, medications that influence the risk of developing urinary stones, and their antidiabetic regimens, using diagnostic codes specified in online supplementary table 1. The Charlson comorbidity index (CCI) was calculated using methods described previously. The severity of DM was estimated using the adapted diabetes complications severity index (aDCSI), consistent with prior reports.7
Supplemental material
These patients were prospectively followed from the index date, defined as the day when participants satisfied the criterion of DM diagnosis, until the development of urolithiasis, mortality, or the end of the study period (December 31, 2011).
Variables and outcome
The primary exposure of this diabetic cohort was the existence and severity of frailty. We defined frailty using a modified version of the FRAIL scale, a widely used frailty-assessing instrument proposed by the International Association of Nutrition and Aging. The applicability of the FRAIL scale in patients with diabetes has been demonstrated in different populations, including Asian and domestic ones.9 10 The original FRAIL scale was created based on responses to five self-reported items, namely: fatigue, resistance, ambulation, illness, and loss of body weight;11 the scoring results of the FRAIL scale have been extensively found to correlate with medical outcomes, including disability, healthcare utilization, costs, and overall survival.7 12 The convenience of the FRAIL scale has facilitated its use as a rapid frailty screening tool in clinical settings. We further adapted the FRAIL scale by incorporating combinations of diagnostic code groups for each of its five items, as published previously.7 This approach has been repeatedly validated, and scoring results based on this modified FRAIL scale exhibited excellent correlations with patient-level outcomes.7 8 Patients with any code from the diagnostic groupings during the years preceding the index date were deemed to exhibit positivity for that specific FRAIL item. We defined patients with at least three positive items as having frailty, according to the original scheme of FRAIL scale. The severity of frailty was recorded during follow-up for analytic purposes.
The outcome of this study was the development of incident urolithiasis, including either upper (renal) or lower urinary tract (ureteral and bladder) stones. Incident stone formation was recognized using previously published ICD-9-CM codes:13 592.x (calculus of the kidney), 274.11 (uric acid nephrolithiasis), and 594.x (calculus of the lower urinary tract). Patients with at least three outpatient diagnoses of stones or at least one inpatient diagnosis during the entire follow-up period were classed as having incident stones, and follow-up was terminated on the earliest date of diagnosis. The use of ICD-9-CM codes from administrative data for identifying urinary tract stones has been previously reported to exhibit sufficient validity.14 15 We further stratified these diagnostic codes into upper (592.x and 274.11) and lower urinary tract (594.x) origin. We also defined patients with recurrent urolithiasis as those with more than three diagnoses of urolithiasis “per year” during follow-up.13
Statistical analysis
We described continuous variables in mean and standard deviation (SD) and compared between groups using the Student’s t-test. Categorical variables were described as numbers with percentages, and groups were compared using the χ² test. For comparisons between more than two groups, we used the one-way analysis of variance.
We first summarized clinical data of study participants without and with 1, 2, or ≥3 FRAIL items at baseline among the entire diabetic cohort and compared these parameters between the four groups. We also identified the proportions of each positive item among the studied participants. After follow-up, we recorded the incidence of urolithiasis in each group of study participants, analyzed the data using the Kaplan-Meier technique, and compared groups using a log-rank test. Cox proportional hazard regression was subsequently used to analyze the relationship between the severity of frailty and the incidence of urolithiasis among patients with diabetes, incorporating demographic data (age ≥65 or not; gender), lifestyle factors, the year of DM diagnosis, comorbidities, changes of frailty status during follow-up, and medications with potential influences on urolithiasis risk, with the risk adjusted for the risk competition of mortality at follow-up.
We further arranged sensitivity analyses focusing on whether the observed relationships differed between stones of different anatomical areas or if the relationship persisted if we analyzed patients with recurrent stone formation.
Results
After using a strict diagnosis of DM and applying our exclusion criteria to 840 000 patients from the Longitudinal Cohort, we identified 525 368 patients with diabetes for analysis in this study (figure 1). Among them, 338 121 (64.4%) did not have FRAIL-recognized frailty, while 149 748 (28.5%), 34 463 (6.6%), and 3 036 (0.6%) had 1, 2, and ≥3 FRAIL items at baseline, respectively. Patients with diabetes with increasing numbers of positive FRAIL items, or more severe frailty, were of a significantly higher age, more likely to be female, and had the complicating lifestyle factors of smoking or alcoholism (all p<0.001) compared with those without or with milder frailty (online supplementary table 2). Patients with DM and more severe frailty had significantly higher CCI scores, more severe diabetes, and a significantly higher prevalence of all comorbidities, including hypertension, liver disease, chronic obstructive pulmonary disease, chronic kidney disease, cardiac disorders, cerebrovascular disease, peripheral vascular disease, malignancy, and rheumatologic illnesses than those without or with a milder degree of frailty (all p<0.001; online supplementary table 2). Patients with diabetes and different severities of frailty also differed significantly with regard to their use of cardiovascular medications (antihypertensives, diuretics, antilipidemic drugs), analgesics (nonsteroidal anti-inflammatory agents, cyclooxygenase 2 inhibitors), gout medications, anticoagulants, antacids (magnesium and calcium), potassium citrate, vitamin D, and bisphosphonates (online supplementary table 2). However, patients with DM and more severe frailty were less likely to have obesity than those without or with mild frailty (p=0.01).
A flowchart of study participant enrollment and categorization. DM, diabetes mellitus; OPD, outpatient department.
Among the 187 247 (35.6%) diabetic patients with mild to severe frailty, the most common FRAIL items were illness (70.5%), followed by fatigue (43.1%), weight loss (3.5%), and resistance (3.2%; online supplementary table 3). For those with only one FRAIL item, 64.4% had illness, followed by fatigue (32.2%) and weight loss (1.6%), and the percentage of patients displaying each FRAIL item increased progressively with higher numbers of FRAIL items.
After an average of 4.2 years of follow-up, 18 034 patients (3.4%) developed at least one episode of incident urolithiasis, of which 16 747 (92.9%) had renal stones and 1742 (9.7%) had lower urinary tract stones. Among those without frailty at baseline, 20.7%, 2.9%, and 0.2% exhibited 1, 2, and ≥3 frailty items during follow-up (online supplementary table 4). Among those with 1 and 2 frailty items at baseline, 1.1% and 6.8% developed full-blown frailty during follow-up (online supplementary table 4). The incidence of urolithiasis among patients with DM increased progressively with higher severities of frailty, from 7.7 cases per 1000 patient-year (non-frail) to 12.8 cases per 1000 patient-year (≥3 FRAIL items) (figure 2), equivalent to an unadjusted hazard ratio (HR) of 14% risk increase per FRAIL item. Cox proportional hazard regression analyses showed that patients with DM having 1, 2, or ≥3 FRAIL items exhibited a significantly higher risk of developing urolithiasis compared with non-frail individuals with diabetes (for 1, 2, and ≥3 FRAIL items; HR1.04, 1.23, and 1.45; 95% confidence interval (CI) 0.99 to 1.10, 1.12 to 1.35, and 1.12 to 1.89, respectively). This effect was independent of age category, gender, the year of DM diagnosis, lifestyle factors, comorbidities, aDCSI, frailty status changes during follow-up, and medications that might influence the risk of developing urolithiasis (table 1). The risk did not change further if we added as a variable the specific types of oral antidiabetic medications. We further analyzed which FRAIL item correlated with an increased risk of urolithiasis and found that the illness item emerged as a significant risk predictor (table 1).
Kaplan-Meier curves for the hazard of developing urolithiasis during follow-up.
Cox proportional hazard regression with primary outcome of incident urolithiasis as the dependent variable
Sensitivity analyses were conducted to examine whether the relationship between frailty and urolithiasis varied according to outcome definitions. We discovered that patients with DM having 1, 2, or ≥3 FRAIL items exhibited a significantly higher risk of developing renal stones compared with non-frail diabetic ones (for 1, 2, and ≥3 FRAIL items; HR: 1.05, 1.27, and 1.47; 95% CI 0.998 to 1.11, 1.15 to 1.41, and 1.10 to 1.96; respectively; table 2). The degree of risk elevation became less prominent if we focused on the risk of developing lower urinary tract stones. On the other hand, the degree of risk increased further if we focused on cases of recurrent urolithiasis during the study period, suggesting that the risk of urolithiasis has a dose-dependent relationship with frailty (table 2).
Sensitivity analyses
Discussion
In this study based on a large diabetic cohort, we discovered that those with frailty at baseline had a significantly higher risk of developing urolithiasis over 4.2 years of follow-up than those without frailty, with the increased urolithiasis risk paralleling frailty severity. The risk of urolithiasis was more prominent for stones of the upper urinary tract. Our findings thus imply that frailty, as a degenerative trait that occurs prematurely in patients with diabetes, plays an under-recognized role in modulating their likelihood of developing urolithiasis in the future.
Wide variation in the prevalence and incidence of urolithiasis has been observed in the literature, depending on geographic region, ethnicity, gender, age, dietary and fluid intake issues, and metabolic disorders or comorbidities, but the overall trend of the incidence of urolithiasis is rising. Based on analyses of the NHANES database, 10.6% of male and 7.1% of female patients in the USA may have nephrolithiasis, which is a marked increase over figures obtained decades ago.16 A longitudinal, general population-based cohort study spanning more than two decades in Sweden reported that the overall incidence of urolithiasis was 0.78 cases per 1000 patient-year, with cases recognized based on ICD codes.17 Estimates from Asian countries are generally higher than those from Western ones; studies involving >120 000 middle-aged or older adults from China showed that the overall incidence of urolithiasis was 2.1–3.8 cases per 1000 patient-year.18 Data from a random sampling of individuals from South Korea yielded an even higher incidence of urolithiasis, up to 5.6 cases per 1000 patient-year during one decade.19 In this study, we identified an elevated risk of urolithiasis among diabetic individuals without and with frailty, between 7.7 and 12.8 cases per 1000 patient-year (table 1), mildly higher than that in other Asian countries, but similar to incidences reported by other local studies (12–13 cases per 1000 patient-year).20 It has been suggested that higher ambient temperature, acidic urine related to rice and increasing protein consumption, abundance of oxalate-containing local food, and a rising prevalence of hyperuricemia may underlie the significantly higher incidence of urolithiasis in the Taiwanese population, and DM may serve as another risk factor. We further identified a previously unrecognized risk factor for urolithiasis, frailty, in the diabetic population, which warrants special attention in aging individuals.
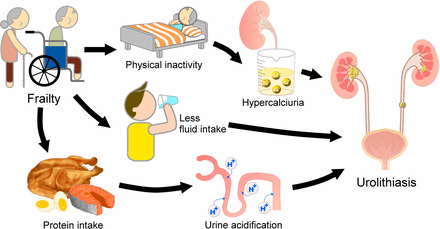
The relationship between frailty and the increased risk of urolithiasis has not been previously reported, and we propose several plausible reasons for this association (figure 3). First, by definition, frail individuals have a significantly lower physical activity compared with non-frail individuals, and a decreased physical activity with immobilization has been recognized as a risk factor for hypercalciuria.21 A sedentary lifestyle or, at its extreme, prolonged bed rest, is not uncommon for frail individuals since their physical capabilities decline and ambulation is frequently compromised. This is further compounded by a higher incidence of peripheral vasculopathy and other cardiac morbidities in patients with DM, as with our participants. Immobilization induces increased bone turnover and leads to resorptive hypercalciuria.22 Second, frail individuals are often encouraged to increase their nutrient intake to avoid negative protein balance,23 and this high dietary protein intake significantly increases the probability of calciuria and renal stones.24 The association between protein intake and higher urine calcium excretion presumably stems from acid production during protein metabolism that buffers via bone mineral mobilization, along with enhanced intestinal calcium absorption, and an elevated glomerular filtration rate.25 Finally, DM per se frequently predisposes patients to fluid loss through osmotic diuresis, and frail individuals, especially older ones, are at an even higher risk of dehydration compared with non-frail ones due to decreased fluid intake and delayed identification of thirst.26 We believe that all of these factors contribute to the observed higher risk of urolithiasis in diabetic individuals with frailty, although more data are needed for confirmation.
A putative diagram illustrating the intricate connections between frailty and the risk of urolithiasis.
In this study, we demonstrated that increasingly severe frailty was a significant predictive factor for recurrent urolithiasis, a finding that has not been previously reported (table 2). Prior studies showed that recurrent urolithiasis was largely mediated by persistent hypercalciuria and/or hypocitraturia,27 and, as explained above, frailty may be an under-recognized etiology for persistent hypercalciuria in susceptible patients. Consequently, it is highly likely that being frail places the affected individuals at risk of developing recurrent urolithiasis. In addition, chronic metabolic disorders, such as gout or hypertension, and multimorbidity, which is common in patients with diabetes and the elderly, have been shown to elevate the risk for urolithiasis,28 29 which was previously attributed to elevated systemic and local oxidative stress.30 We believe that frailty can be another plausible mechanism predisposing patients with chronic metabolic disorders to the development of urolithiasis, and strategies aiming at ameliorating urinary stone may have to consider the contribution of frailty.
Management of symptomatic urolithiasis includes surgical or percutaneous endoscopic lithotripsy and shockwave or medical expulsive therapy, but for asymptomatic stone carriers, dietary interventions, including salt reduction, normalizing calcium and protein, and appropriate fluid intake are integral chronic care components. In light of our findings, interventions directed toward ameliorating frailty, especially exercise programs, among frail patients with DM are expected to further lower the risk of urolithiasis, in conjunction with the dietary modifications outlined above. Interventions toward frailty reduction are expected to be viable options among the therapeutic armamentarium for urolithiasis.
Our study benefited from the diabetic cohort used, the LCDP, that encompassed a representative, nationwide sampling of patients with DM, and the results generated from the analysis of this cohort are enhanced by its comprehensive data documentation and longitudinal nature.7 8 31 Furthermore, the modified FRAIL scale used in this study has been validated before in this database7 8 and other cohorts,32 33 and the code combinations for identifying urolithiasis were also derived from studies using administrative database to uncover cases of urolithiasis. However, several limitations should be noted; frailty was not ascertained using interview results, and extrapolation of our findings to the individual level should proceed cautiously. Unmeasured confounders might still exist. We evaluated only patients with diabetes, so whether the relationship holds true in those with other comorbidities or the general population remains unclear. We did not collect information related to dietary variations, exercise habits, and fluid intake in this cohort, so there might still be residual confounders in the results; nonetheless, we believe that the large number of cases in our cohort is expected to balance these unidentified confounders. A single assessment of frailty may have limited value for outcome prediction. Cases of diagnostic code-identified urolithiasis have not been verified in this cohort. Finally, our findings have not been verified in another independent cohort. Further replicative study is needed to confirm the relationship we observed.
Conclusion
Patients with diabetes are at a higher risk of having frailty and developing urolithiasis, and we examined the relationship between frailty and incident urolithiasis in these patients. We discovered that having frailty was a significant predictor of developing urolithiasis in the future, with the probability of stone formation increasing stepwise with the severity of frailty. Based on our findings, treatment against frailty may be a potential approach for reducing the risk of developing first-time and recurrent urolithiasis in patients with DM. Prospective cohort studies are warranted to confirm these findings, and we are currently in the process of designing an appropriate study.
Acknowledgments
We are grateful to the second core laboratory, Department of Medical Research of National Taiwan University Hospital for their technical assistance.
References
Footnotes
Contributors Study design: C-TC, JW, J-WH. Data analysis: C-TC, K-YH, JW. Article drafting: C-TC, JW, J-WH, K-YH, K-LC. All authors approved the final version of the manuscript.
Funding The study is financially sponsored by National Taiwan University Hospital and Ministry of Science and Technology, Taiwan (MOST 108-2314-B-002-055-).
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval The protocol of the current study was approved by the institutional review board of the National Taiwan University Hospital (NO. 201802063W), and its content adhered to the Declaration of Helsinki. Informed consent was waived for all participants as adjudicated by the review board.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement The data used to conduct this study are not for release per administrative regulation, but additional analystic results will be available on reasonable request.