Abstract
Aims/hypothesis
We examined whether type of diabetes and/or insulin treatment can modulate the impact of sustained hyperglycaemia and glycaemic variability as activators of oxidative stress.
Methods
This was an observational study in 139 patients with diabetes, 48 with type 1, 60 with type 2 treated by oral hypoglycaemic agents (OHAs) alone and 31 with type 2 treated with insulin plus OHAs. In addition, two groups of ten patients with type 2 diabetes were investigated either before and after introducing insulin treatment (add-on insulin group) or before and after add-on OHA therapy to metformin (add-on OHA group). Oxidative stress was estimated from 24 h urinary excretion rates of 8-isoprostaglandin \( {{\text{F}}_{2\alpha }}\left( {8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }}} \right) \). HbA1c was assessed and mean amplitude of glycaemic excursions (MAGE) was estimated by continuous monitoring.
Results
The 24 h excretion rate of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) (median [range] picomoles per millimole of creatinine) was much higher (p < 0.0001) in type 2 diabetes patients treated with OHAs alone (112 [26–329]) than in the type 1 diabetes group (65 [29–193]) and the type 2 diabetes group treated with insulin (69 [30–198]). It was associated with HbA1c (F = 12.9, p = 0.0008) and MAGE (F = 7.7, p = 0.008) in non-insulin-treated, but not in insulin-treated patients. A significant reduction in 24 h excretion rate of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) from 126 (47–248) to 62 (35–111] pmol/mmol of creatinine was observed in the add-on insulin group (p = 0.005) but not in the add-on OHA group.
Conclusions/interpretation
In type 1 and type 2 diabetes, insulin exerts an inhibitory effect on oxidative stress, a metabolic disorder that is significantly activated by sustained hyperglycaemia and glucose variability in non-insulin-treated type 2 diabetes.
Similar content being viewed by others
Introduction
Protein glycation, activation of oxidative stress and inflammation are major causes of diabetic complications in persons with type 1 or type 2 diabetes [1, 2], with both fasting and postprandial hyperglycaemia contributing to the overall glucose exposure [3–5]. However, an increasing body of evidence is emerging that supports including glycaemic variability as an additional risk factor for diabetic complications [6, 7]. A few years ago, Mohanty et al. [8] reported that an acute glucose challenge results in increased generation of reactive oxygen species (ROS) by leucocytes. In addition, several studies [9, 10] in type 2 diabetes indicate that glucose fluctuations are potent activators of oxidative stress, a key player in the development of cardiovascular diseases in diabetes [1, 2]. However, this is in contrast to observations made in type 1 diabetes patients [11, 12]. Thus Kilpatrick et al. [11] reported that sustained hyperglycaemia was predictive of microvascular complications in patients with type 1 diabetes, while glycaemic variability was not. Revisiting DCCT data, Lachin et al. [13] concluded that glucose variability only explained a small part of the differences between intensive and conventional treatment groups. In short, sustained hyperglycaemia is a well established risk factor for diabetic complications, while the role of glycaemic variability continues to be debated [7].
The apparent discrepancies between type 1 and type 2 diabetes raise the question of whether insulin as such could regulate oxidative stress in diabetes. To gain further insight into the above-mentioned questions, we compared the urinary excretion rate of 8-isoprostaglandin \( {{\text{F}}_{2\alpha }}\left( {8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }}} \right) \) [14, 15], a reliable marker of oxidative stress, in three groups of patients, one with type 1 diabetes, one with type 2 diabetes treated by oral hypoglycaemic agents (OHAs) alone and the last with type 2 diabetes treated by a combined therapy (OHAs plus insulin). Drawing on the demonstration by Dandona et al. [16] that insulin inhibits ROS generation and p47phox expression in mononuclear cells of obese patients, we hypothesised that insulin as such could exert an inhibitory effect on oxidative stress. We therefore investigated an additional group of patients with type 2 diabetes, estimating their 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) before and after initiation of insulin treatment.
Methods
Patients
A total of 139 persons with diabetes in Montpellier, France, were included in the study between 2003 and 2009. Of these, 48 had type 1 diabetes and 91 type 2 diabetes treated with OHAs alone (n = 60) or in combination with insulin (n = 31). Types of treatment and insulin doses are detailed in Table 1. Basal insulin regimens were provided as a once daily injection of insulin glargine. Basal–bolus insulin regimens consisted of a once daily injection of insulin glargine, combined with pre-meal boluses of rapid-acting insulin analogues given once, twice or thrice daily as appropriate. The participants were entered consecutively without any selection based on HbA1c, age, sex or diabetic complications. All patients with type 1 diabetes had been treated with insulin from diagnosis of the disease (Table 1). All patients with type 2 diabetes had been treated with a stable combination of OHAs or OHAs and insulin for at least 3 months prior to entry into the study (Table 1). No one was excluded on the basis of categories of OHAs or types of insulin regimen. Exclusion criteria included patients who had experienced an acute concurrent illness or been treated with steroids or non-steroid anti-inflammatory drugs (except aspirin) during the preceding 3 month period and any individual who had not ceased smoking habits for at least 1 year or who had a glomerular filtration rate of less than 60 ml min−1 1.73 m−2 using the Cockcroft–Gault formula [17]. To further evaluate the possible effect of insulin treatment on oxidative stress, an additional ten persons with type 2 diabetes were investigated before and after introduction of insulin treatment (add-on insulin group). This group was compared with ten type 2 diabetes patients who had initially been treated with metformin alone (2 g/day) and in whom the treatment had been reinforced for 3 months either with glimepiride (1 to 4 mg/day, seven patients) or rosiglitazone (4 to 8 mg/day, three patients). These patients (add-on OHA group) were matched for 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) at baseline with the add-on insulin group.
The study was observational in design. All investigations, including continuous glucose monitoring and urine collections for \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \), were routinely performed in the diabetic patients admitted to our outpatient clinic. Diabetes management was handled by participants together with their usual healthcare provider. As a consequence, each participant gave informed, but not written informed consent. In accordance with the directives of article L-1121-1 of the French Code for Public Health concerning Biomedical Research [18], the study design did not require approval from local Ethics Committees. Control data (n = 23) were retrospectively drawn from a vitamin E study in healthy individuals, conducted in 2001 at the University of Montpellier, France (E. Mas, F. Michel and J. P. Cristol, unpublished results).
Study protocol
Subcutaneous interstitial glucose was monitored on an ambulatory basis over a period of three consecutive days using the second generation of a continuous glucose monitoring system (Minimed, Northridge, CA, USA) [19, 20]. The sensor was inserted on day 0 and removed on day 3 at mid-morning. Calculations of glucose variations were averaged from data obtained on days 1 and 2 in order to avoid bias due to insertion and removal of the sensor. During this period, each patient was asked to collect a 24 h urinary sample that was stored at −80°C until analysis for \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) and creatinine. On day 0, at breakfast time, after an overnight fast and before sensor insertion, venous blood samples were drawn into tubes containing EDTA. Plasma was separated from the cells within 1 h and assayed for creatinine, glucose, HbA1c, total cholesterol, HDL-cholesterol and total triacylglycerol. Systolic and diastolic blood pressures were determined on the right arm after at least 5 min rest in the supine position.
Laboratory measurements
Plasma glucose concentrations were measured by a hexokinase method using a glucose analyser (Synchron CX4/CX5; Beckman Instruments, Fullerton, CA, USA). Both the intra- and inter-assay coefficients of variation were ≤2% at values of less than 7 mmol/l. HbA1c was measured using a high-performance liquid chromatography assay (Menarini Diagnostics, Florence, Italy). The intra-assay and inter-assay coefficients of variation were less than 3% at values of less than 8%. HbA1c determinations were DCCT-aligned. Total cholesterol was determined on an analyser kit (PRO Konelab; Thermo Electron, Vantaa, Finland) by a cholesterol oxidase method. Triacylglycerol was measured on the same analyser by an enzymatic method using an automated glycerol blank. HDL-cholesterol was quantified without any pre-treatment using polyethylene glycol-modified enzymes and dextran sulfate and a kit from Thermo Electron. LDL-cholesterol concentrations were calculated from measurements of total cholesterol, triacylglycerol and HDL-cholesterol concentrations using the formula of Friedewald et al. [21]. Plasma and urinary creatinine levels were determined by an enzymatic method (Randox Laboratories, Crumlin, Wales, UK) and the Jaffe kinetic method for plasma and urine, respectively.
Free \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \), the most frequently measured F2 isoprostane in body fluids, was determined using an enzyme immunoassay method (SPI-BIO; Cayman Chemical, Montigny-le-Bretonneux, France). Purification and extraction of urine samples were performed before assay as previously described [9]. The intra- and inter-assay CVs were 3% and 3.4%. The 24 h urinary excretion rate of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) was expressed as picomoles per millimole urinary creatinine. As commonly reported in all publications [14, 15, 22, 23], concentration ratios were used to minimise the consequences of inadequate urine collection over 24 h. This adjustment, moreover, has been shown to suppress the difference between men and women in urinary excretion rates of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) [24].
Assessment of glycaemic variability
The mean amplitude of glycaemic excursions (MAGE) [25, 26] was used to assess glucose fluctuations from the 24 h glucose profiles obtained by continuous glucose monitoring as previously described [9].
Statistical analysis
Analysis of variance (or covariance), Kruskal–Wallis test and χ 2 test were used, as appropriate, to compare the different groups of patients. Statistical comparisons were considered significant at p < 0.05. Multiple testing was corrected using Bonferroni correction or the Mann–Whitney test as appropriate. The Wilcoxon paired test was used for comparing data at baseline and at the end of the follow-up period in the two groups of ten patients in whom the reinforcement of treatment had consisted of either insulin or an additional OHA respectively. Univariate analysis was done by using simple linear correlations and by determining the Pearson correlation coefficient r. Multivariate analysis of covariance was used to assess the independent effects of markers of sustained hyperglycaemia (HbA1c and 24 h mean glucose) and glycaemic variability (MAGE) on 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \). Adjustments were made for age, sex, BMI, categories of treatments and presence or absence of diabetic complications. Given the strong correlation between HbA1c and 24 h mean glucose concentration, two models were tested: the first included HbA1c and the second 24 h mean glucose concentrations. Analysis was performed with the Statview version 5 for Macintosh (SAS Institute, Cary, NC, USA).
Results
Results are given as medians (ranges). Clinical and laboratory characteristics of study participants are described in Table 1. In addition, the 23 non-diabetic controls (15 men, 8 women) were 66 (54–80) years old, with a BMI of 25.5 (19.4–32.3) kg/m2 and a 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) of 75 (56–142) pmol/mmol of creatinine. Other values included: resting BP, measured as systolic BP 131 (60–154) mmHg and diastolic BP 80 (58–110) mmHg; plasma creatinine 74 (50–102) µmol/l; and plasma lipids, i.e. total cholesterol 5.46 (3.65–6.86) mmol/l, HDL-cholesterol 1.45 (0.91–2.39) mmol/l and total triacylglycerol 1.25 (0.61–3.55) mmol/l.
Variables of glycaemic control in the three groups of participants with diabetes
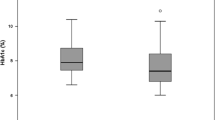
HbA1c levels and mean glucose concentrations over 24 h (Table 1) were comparable in the three groups. MAGE indexes were significantly higher in patients with type 1 diabetes than in patients with type 2 diabetes treated with either OHAs (p < 0.0001) or insulin (p = 0.0022; Fig. 1).
HbA1c levels (a), MAGE indexes (b) and 24 h urinary excretion rates of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) (c) in patients with (white boxes) type 1 diabetes, (grey boxes) type 2 diabetes treated with OHAs and (dark grey boxes) type 2 diabetes treated with OHAs plus insulin. Results are median with interquartile range, 10th and 90th percentiles. †p < 0.0001 and ‡p = 0.0022, ANOVA or Kruskal–Wallis test as appropriate
Urinary excretion rates of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) in the three groups of participants with diabetes
The 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) was different in the three groups even after adjustment for age, sex, BMI and HbA1c (Fig. 1). Despite lower MAGE values, 24 h \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) was significantly higher (p < 0.0001) in the 60 type 2 diabetes patients treated with OHAs alone than in the 48 patients with type 1 diabetes or the 31 type 2 diabetes patients treated with additional insulin therapy. In the 23 non-diabetic controls, mean 24 h \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) was lower than in type 2 diabetic patients treated with OHAs alone (p = 0.0001 after adjustment for age, sex and BMI), but no difference was observed between controls and type 1 or type 2 study participants treated with insulin.
Relationships between urinary excretion rates of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) and markers of metabolic control and diabetic complications
Univariate analysis
In persons with type 2 diabetes treated with OHAs alone, 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) was positively and similarly correlated with HbA1c (r = 0.386, p = 0.0021), 24 h mean glucose concentrations (r = 0.330, p = 0.0096) and MAGE (r = 0.386, p = 0.0021). By contrast, these correlations were not found in type 1 diabetic patients (HbA1c r = −0.058, p = 0.70; 24 h mean glucose concentrations r = 0.25, p = 0.08; MAGE r = 0.011, p = 0.94) or in type 2 diabetic participants treated with insulin (HbA1c r = 0.085, p = 0.65; 24 h mean glucose concentrations r = −0.043, p = 0.82; MAGE r = −0.346, p = 0.06). No significant correlation was found between 24 h \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) and all routine markers of lipid metabolism (total cholesterol, total triacylglycerol, HDL-cholesterol, LDL-cholesterol). In addition, there was no significant association with micro- or macrovascular complications.
Multivariate analysis in type 2 diabetes treated with OHAs alone
Multivariate analysis was performed in type 2 diabetic participants treated with OHAs alone to assess the independent effects of sustained chronic hyperglycaemia and glycaemic variability on activation of oxidative stress. As mentioned above, two models were tested. In both models (Table 2), glycaemic variability (MAGE) and variables of sustained hyperglycaemia (HbA1c or 24 h mean glucose concentrations) were tested as independent explanatory variables. The rationale for considering sustained hyperglycaemia and glycaemic variability as independent variables [27] was based on the fact that MAGE was far from any significant correlation with either HbA1c (r = −0.05) or 24 h mean glucose concentrations (r = 0.134). In the first model, 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) was strongly associated with HbA1c and MAGE, the association being stronger with HbA1c (F = 12.9, p = 0.0008) than with MAGE (F = 7.7, p = 0.008). In the second model, 24 h \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) was associated with 24 h mean glucose concentrations (F = 5.2, p = 0.03) and MAGE (F = 4.4, p = 0.04), but the significance of the association was less than that in the first model. In models 1 and 2, other factors such as age, sex, BMI, categories of treatments for conditions other than diabetes (antihypertensive, lipid-lowering and anti-aggregant treatments) and presence or absence of micro- or macrovascular disease were never found to be associated with 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \). In Fig. 2, 24 h \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) was stratified by the medians of HbA1c and MAGE, clearly showing that the higher the HbA1c and MAGE value, the greater the 24 h urinary excretion rates of \( {\text{PG}}{{\text{F}}_{2\alpha }} \).
Bar graph of 24 h urinary excretion rates of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) in patients with type 2 diabetes treated with OHAs. Patients were divided into two groups with HbA1c lower (n = 28) or higher (n = 32) than median (8.2%). In each group, patients were separated into two subsets, with MAGE below (white columns) or above (black columns) the median (4.3 mmol/l), respectively. Range corresponds to minimum and maximum values
Comparison of urinary excretion rates of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) in participants with type 2 diabetes before and after initiation of insulin treatment (add-on insulin group)
In ten participants with type 2 diabetes, 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) was evaluated while on treatment with OHAs alone and at 10 (3–48) months after initiation of insulin treatment (Table 3, Fig. 3a). The clinical characteristics of this group are depicted in Table 3. A drastic reduction was observed, from 126 (47–248) to 62 (35–111) pmol/mmol of creatinine (p = 0.005; Fig. 3a). No significant changes were observed for MAGE values or HbA1c. The absence of significant improvement in HbA1c can be explained by the fact that the maximal time-interval between initiation of insulin treatment and the investigation on insulin was 4 years. In the insulin-treated group of the UKPD Study, it was shown that after a transient improvement in diabetic control, HbA1c levels returned to baseline a few years after initiation of insulin treatment [28]. However, this explanation is not compelling, and it is more likely that the lack of improvement in HbA1c was partly due to the fact that the patients were submitted to a non-treat-to-target insulin regimen with a freely conducted design as mentioned above. Whatever the reason for the lack of change in HbA1c, the fact remains that the 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) was halved.
a Urinary excretion rates (24 h) of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) before and after initiation of insulin treatment in ten type 2 diabetic patients treated with OHAs (add-on insulin group). p = 0.005 for comparison between baseline and insulin treatment (Wilcoxon’s paired test). b Results as above (a) for ten patients treated with metformin alone before and after add-on therapy with glimepiride or rosiglitazone (add-on OHA group). Comparison between baseline and add-on therapy (Wilcoxon’s paired test) was not statistically significant
Comparison of urinary excretion rates of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) in participants with type 2 diabetes before and after add-on therapy to metformin with a second OHA
The main clinical characteristics of these participants are described in Table 3. No significant changes were found for 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) between baseline (109 [58–228] pmol/mmol creatinine) and the end of the 3-month period of follow-up on a second OHA (113 [75–203] pmol/mmol; Fig. 3b).
Discussion
Our results indicate that in non-insulin-treated persons with type 2 diabetes, 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) was positively and similarly dependent on HbA1c and MAGE indexes. However, in insulin-treated diabetes patients (type 1 and type 2), this relationship disappeared and 24 h \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) was within the normal range and half that of non-insulin-treated type 2 diabetic patients. In a small group of type 2 diabetes patients on OHAs, the 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) was significantly reduced by more than 50% after implementation of insulin add-on therapy. In contrast, 24 h \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) remained unchanged when an OHA (sulfonylurea or thiazolidinedione) was added to metformin in patients not sufficiently controlled on metformin alone.
Considered as a whole, these results question the respective roles of dysglycaemia and insulin therapy in the activation of oxidative stress. They confirm that activation of oxidative stress is increased among non-insulin-treated type 2 diabetes patients compared with non-diabetic individuals [22, 23, 29]. Even though the oxidative stress excursions depend on acute changes in glucose concentrations [9, 10, 30], sustained hyperglycaemia and glucose variability seem to contribute equally to the activation of the oxidative stress. The current results partially confirm those observed in a previous preliminary study [9], in which we reported that 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) was positively and highly correlated with MAGE index alone, but not with HbA1c. However, this strong relationship was observed in a small group of 21 participants with type 2 diabetes, who exhibited more loosely controlled diabetes (mean HbA1c 9.6%) than those investigated in the present study. Here, both in type 1 and type 2 diabetes patients treated with insulin, 24 h \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) was normal or near normal. Furthermore, the mean 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) dropped from elevated to normal values when non-insulin-treated participants with type 2 diabetes were switched to insulin add-on therapy. In contrast, mean 24 h \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) remained elevated in type 2 diabetes patients after reinforcement of metformin treatment with rosiglitazone or glimepiride. These results are in agreement with those found by Vesby et al. [23], who did not observe any significant increase in 24 h \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) among patients with type 1 diabetes. They also bridge the divide between our previous observations in participants with type 2 diabetes on OHAs [9] and those reported in type 1 diabetes by several author groups, who concluded that glycaemic variability does not appear to play a role as risk factor for microvascular complications [11, 13] or to activate oxidative stress among these participants [12].
All diabetic participants included in the present study had similar levels of HbA1c and glucose variability on a decreasing ordinal scale: type 1 diabetes > insulin-treated type 2 diabetes > non-insulin-treated type 2 diabetes. Differences between the latter and the other two groups were significant. Consequently, it is likely that the differences in 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) between the three groups are not due to dysglycaemia represented by HbA1c or glycaemic variability. The small but not significant differences observed between the three groups in terms of age and plasma lipid concentrations seem to exclude such factors as explanations of the differences in 24 h \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \). Furthermore factors like BMI, treatments with antihypertensive, lipid-lowering or anti-aggregant agents and presence or absence of micro and macrovascular disease did not seem to have any impact upon the activation of oxidative stress, since the multivariate analysis indicated that 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) did not show any statistical association with the factors mentioned above. Although obesity is undoubtedly associated with oxidative stress [31, 32], hyperglycaemia and insulin seem to exert more powerful effects. We therefore suggest that insulin treatment as such is the main determinant of the difference between participants who received insulin treatment and those who did not. This explanation seems to be supported by the normalisation of 24 h urinary excretion of \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) when insulin treatment was implemented in persons with type 2 diabetes.
Given these observations, the administration of exogenous insulin appears to exert a strong inhibitory effect on activation of oxidative stress, a hypothesis that is in agreement with its anti-inflammatory effect [33, 34]. For instance, insulin added to in vitro preparations of vascular smooth muscle cells extracted from aortas of rats was shown to inhibit activation of nuclear factor-κB [35], a pro-inflammatory transcription factor activated by glucose [36]. The first demonstration of the anti-inflammatory effect of insulin in vitro was in human aortic endothelial cells at physiologically relevant concentrations of insulin [37]. A first demonstration that insulin exerts a suppressive effect on ROS, p47phox and nuclear factor-κB in humans in vivo was carried out in circulating mononuclear cells [16]. This observation is relevant to the data presented here since \( 8 - {\text{iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) is formed by lipid peroxidation by ROS. In animal models insulin exerts a direct inhibitory effect on endotoxin-induced inflammation [38]. In clinical trials, insulin infusions resulted in 40% reductions in plasma C reactive protein concentrations either in patients with acute myocardial infarction [39] or in patients undergoing coronary artery bypass grafts [40].
Thus our own data and those reported by other authors provide an accumulating bulk of evidence for both an anti-oxidant and anti-inflammatory action of insulin. Both effects would overcome the deleterious pro-oxidant and pro-inflammatory action of glycaemic disorders, which include sustained hyperglycaemia and acute glucose fluctuations. Furthermore, insulin can up- and downregulate the expression of genes involved in many metabolic pathways [41]. As a result, insulin is potentially able to exert regulatory effects upon the biochemical mechanisms implicated in oxidative stress. However, such potential effects remain controversial because most were established using experimental conditions [42] that are remote from those in real-life conditions, which were preserved in the present study.
The data of long-term interventional trials might help confirm the inhibitory action of insulin on oxidative stress, but results to date are somewhat disappointing. In the UKPD Study the risk of diabetes-related deaths and of developing any diabetes-related complication was similar in the three intensively treated groups, regardless of treatment (chlorpropramide, glibenclamide or insulin) [28]. These results do not seem to be in agreement with a putative inhibitory effect of insulin on oxidative stress, but when interpreting them, caution is required for several reasons. First it should be noted that approximately 20% (339/1,573) of the patients initially enrolled for intensive treatment with sulfonylureas were further submitted to an early addition of insulin. Furthermore, patients assigned to insulin gained more weight and suffered more hypoglycaemic episodes than those assigned to sulfonylureas. In the Hyperglycemia and its Effects After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus (HEART2D) Study [43], the inhibitory effect of insulin on activation of oxidative stress might explain why smaller glucose fluctuations with prandial insulin regimens did not improve cardiovascular outcomes compared with basal insulin regimens in type 2 diabetic participants.
In conclusion, our results, obtained in a pragmatic and observational study, suggest that the activation of oxidative stress is dependent upon sustained hyperglycaemia and glycaemic variability only in type 2 diabetic participants treated with OHAs alone and not in insulin-treated diabetic participants. From a pathophysiological point of view, these observations seem to provide a unifying explanation for the discrepancies that have been previously reported between insulin-treated type 1 [11, 12] and non-insulin-treated type 2 diabetes [9]. From a clinical point of view, our data strongly suggest that insulin treatment exerts a beneficial effect by inhibiting the activation of oxidative stress. Consequently, the present study should trigger future trials comparing the effects on diabetic complications of early insulin treatment vs escalation of OHA therapy [44, 45].
Abbreviations
- \( 8 - {\text{Iso}} - {\text{PG}}{{\text{F}}_{2\alpha }} \) :
-
8-Isoprostaglandin \( {{\text{F}}_{2\alpha }} \)
- MAGE:
-
Mean amplitude of glycaemic excursions
- OHA:
-
Oral hypoglycaemic agent
- ROS:
-
Reactive oxygen species
References
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625
Gorus F, Mathieu C, Gerlo E (2006) How should HbA1c measurements be reported? Diabetologia 49:7–10
Sacks DB, Bruns DE, Goldstein DE, Mac Laren NK, McDonald JM, Parrott M (2002) Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus (Position statement). Diabetes Care 25:750–786
Monnier L, Lapinski H, Colette C (2003) Contributions of fasting and postprandial plasma increments to overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care 26:881–885
Hirsch IB, Brownlee M (2005) Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications 19:178–181
Monnier L, Colette C (2008) Glycemic variability. Should we and can we prevent it? Diabetes Care 31(Suppl 2):S150–S154
Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P (2000) Glucose challenge stimulates reactive oxygen species (ROS) generation by leukocytes. J Clin Endocrinol Metab 85:2970–2973
Monnier L, Mas E, Ginet C et al (2006) Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295:1681–1687
Ceriello A, Esposito K, Piconi L et al (2008) Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 57:1349–1354
Kilpatrick ES, Rigby AS, Atkin SL (2006) The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care 29:1486–1490
Wentholt IME, Kulik W, Michels RPJ, Hoekstra JBL, de Vries JH (2008) Glucose fluctuations and activation of oxidative stress in patients with type 1 diabetes. Diabetologia 51:183–190
Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN for the DCCT/EDIC Research Group (2008) Effect of glycemic exposure on the risk of microvascular complications in the Diabetes Control and Complications Trial-Revisited. Diabetes 57:995–1001
Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ (1990) A series of prostaglandin F2-like compounds are produced in vivo in humans by non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A 87:9383–9387
Roberts LJ, Morrow JD (2000) Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med 28:505–513
Dandona P, Aljada A, Mohanty P et al (2001) Insulin inhibits intracellular NFkB and stimulates IkB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab 86:3257–3265
Cockcroft DW, Gault HM (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Code de la Santé Publique (2009) Partie legislative. Première partie. Livre premier. Titre II, Chapitre 1, Article L-1121-1. 18 Avril 2006 (version consolidée du 29 Août 2009). Available from www.legifrance.com, accessed 31 August 2009
Buckingham B, Block J, Wilson DM (2005) Continuous glucose monitoring. Curr Opin Endocrinol Diabetes 12:273–279
Monnier L, Colette C, Boegner C, Pham TC, Lapinski H, Boniface H (2007) Continuous glucose monitoring in patients with type 2 diabetes: why? When? Whom? Diabetes Metab 33:247–252
Friedewald WT, Levy RI, Frederickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifugation. Clin Chem 18:499–502
Patrono C, Falco A, Davi G (2005) Isoprostane formation and inhibition in atherothrombosis. Curr Opin Pharmacol 5:198–203
Vesby J, Basu S, Mohsen R, Berne C, Vesby B (2002) Oxidative stress and antioxidant status in type 1 diabetes mellitus. J Intern Med 251:69–76
Morales CR, Terry ES, Zackert WE, Montine TJ, Morrow JD (2001) Improved assay for the quantification of the major urinary metabolite of the isoprostane 15-F2t-isoprostanes (8-iso-PGF2α) by a stable isotope dilution mass spectrometric assay. Clin Chim Acta 314:93–99
Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF (1970) Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 19:644–655
Service FJ, O’Brien PC, Rizza RA (1987) Measurements of glucose control. Diabetes Care 10:225–237
Derr R, Garrett E, Stacy GA, Saudek CD (2003) Is HbA1c affected by glycemic instability? Diabetes Care 26:2728–2733
UK Prospective Diabetes Study Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352:837–853
Basu S (1998) Metabolism of 8-iso-prostaglandin F2α. FEBS Lett 428:32–36
Ceriello A, Quagliaro L, Catone B et al (2002) Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes Care 25:1439–1443
Dandona P, Mohanty P, Ghanim H et al (2001) The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxydation, and protein carbonylation. J Clin Endocrinol Metab 86:355–362
Davi G, Guagnano MT, Ciabattoni G et al (2003) Platelet activation in obese women: role of inflammation and oxidant stress. JAMA 289:1781–1782
Dandona P, Aljada A, Mohanty P (2002) The anti-inflammatory and potential anti-atherogenic effect of insulin: a new paradigm. Diabetologia 45:924–930
Dandona P, Chaudhuri A, Ghanin H, Mohanty P (2009) Insulin as an anti-inflammatory and antiatherogenic modulator. J Am Coll Cardiol 53:S14–S20
De Oliveira Silva C, Delbosc S, Araïs C, Monnier L, Cristol J-P, Herbute N (2008) Modulation of CD36 protein expression by AGEs and insulin in aortic VSMCs from diabetic and non-diabetic rats. Nutr Metab Cardiovasc Dis 18:23–30
Du X, Stocklauser-Farber K, Rosen P (1999) Generation of reactive oxygen intermediates, activation of NF-kappaB and induction of apoptosis in human endothelial cells by glucose: role of nitric oxide synthase? Free Radic Biol Med 27:752–763
Aljada A, Saadeh R, Assian E, Ghanim H, Dandona P (2000) Insulin inhibits the expression of intercellular adhesion molecule-1 by human aortic endothelial cells through stimulation of nitric oxide. J Clin Endocrinol Metab 85:2572–2575
Horvath EM, Benko R, Gero D, Kiss L, Szabo C (2008) Treatment with insulin inhibits poly (ADP-ribose) polymerase activation in a rat model of endotoxemia. Life Sci 82:205–209
Wong VW, McLean M, Boyages SC, Cheung NW (2004) C-reactive protein levels following acute myocardial infarction: effect of insulin infusion and tight glycemic control. Diabetes Care 27:2971–2973
Visser L, Zuurbier CJ, Hoek FJ et al (2005) Glucose, insulin and potassium applied as perioperative hyperinsulinaemic, normoglycaemic clamp: effects on inflammatory response during coronary artery surgery. Br J Anaesth 95:448–457
Rome S, Clemente K, Rabasa-Lhoret R et al (2003) Microarray profiling of human skeletal muscle reveals that insulin regulates 800 genes during a hyperinsulinemic clamp. J Biol Chem 278:18063–18068
Kuboki K, Jiang ZY, Takahar N et al (2000) Regulation of endothelial constitutive nitric synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation 101:676–681
Raz I, Wilson PWF, Strojek K et al (2009) Effects of prandial vs fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D Trial. Diabetes Care 32:381–386
Nathan DM, Buse JB, Davidson MB et al (2009) Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement of the American Diabetes Association. Diabetes Care 32:193–203
Standl E, Schnell O (2008) Insulin as a first-line therapy in type 2 diabetes. Should the use of sulfonylureas be halted? Diabetes Care 31(Suppl 2):S136–S139
Acknowledgements
This study was supported by Academic funds from the University of Montpellier (France).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monnier, L., Colette, C., Mas, E. et al. Regulation of oxidative stress by glycaemic control: evidence for an independent inhibitory effect of insulin therapy. Diabetologia 53, 562–571 (2010). https://doi.org/10.1007/s00125-009-1574-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-009-1574-6