Abstract
Aims/hypothesis
Endothelium-derived factors are thought to be physiological modulators of large artery stiffness. The aim of the study was to investigate whether endothelial function could be a determinant of arterial stiffness in essential hypertensive patients, in relation with the concomitant presence of type 2 diabetes mellitus.
Methods
The study included 341 participants (84 hypertensive patients with and 175 without type 2 diabetes mellitus, 82 matched controls). Brachial artery endothelium-dependent flow-mediated dilation (FMD) was determined by high-resolution ultrasound and computerised edge detection system. Applanation tonometry was used to measure carotid–femoral pulse wave velocity (PWV).
Results
Hypertensive patients with diabetes had higher PWV (10.1 ± 2.3 m/s vs 8.6 ± 1.4 m/s, p < 0.001) and lower FMD (3.51 ± 2.07 vs 5.16 ± 2.96%, p < 0.001) than non-diabetic hypertensive patients, who showed impaired vascular function when compared with healthy participants (7.9 ± 1.6 m/s and 6.68 ± 3.67%). FMD was significantly and negatively correlated to PWV only in hypertensive diabetic patients (r = −0.456, p < 0.001), but not in hypertensive normoglycaemic patients (r = −0.088, p = 0.248) or in healthy participants (r = 0.008, p = 0.946). Multivariate analysis demonstrated that, in the diabetic group, FMD remained an independent predictor of PWV after adjustment for confounders (r 2 = 0.083, p = 0.003). Subgroup analysis performed in non-diabetic hypertensive patients revealed that neither obesity nor the metabolic syndrome affected the relationship between FMD and PWV.
Conclusions/interpretation
Endothelial dysfunction is a determinant of aortic stiffness in hypertensive diabetic patients but not in hypertensive patients without diabetes. These results suggest that type 2 diabetes mellitus on top of hypertension might worsen arterial compliance by endothelium-related mechanisms.
Similar content being viewed by others
Introduction
Arterial stiffness has been commonly considered to be a consequence of structural alterations of the vessel wall. However, mechanistic studies have suggested that a ‘functional’ component may contribute to the compliance of large arteries. In particular, endothelium-derived factors such as NO [1] and endothelin-1 [2] have been proposed as physiological modulators of arterial stiffness in healthy individuals. An inverse correlation between endothelial dysfunction and arterial stiffness has been reported in cross-sectional studies performed in mixed cohorts including healthy participants as well as patients with isolated systolic hypertension [3], type 2 diabetes [4–6] and coronary artery disease [7–9], although conflicting results were obtained in healthy participants [10, 11]. Preliminary studies have suggested that vascular correlates of arterial stiffness measurements might differ in patients with cardiovascular risk factors in comparison with healthy individuals [12], also depending on atherosclerotic burden [9], so that studies mixing different populations might be misleading.
Arterial hypertension and type 2 diabetes mellitus are well known cardiovascular risk factors, often associated [13], and both characterised by increased arterial stiffness and endothelial dysfunction [5, 14–16]. Indeed, patients with both hypertension and diabetes mellitus seem to have a stiffer aorta compared with patients with only one of these conditions [17]. Several specific pathophysiological mechanisms are involved in the development of arterial stiffness in type 2 diabetes mellitus, with endothelial dysfunction playing a relevant role [14].
Thus, the aim of the study was to investigate whether conduit artery endothelial function could be a determinant of arterial stiffness in patients with arterial hypertension, and whether the concomitant presence of metabolic alterations such as type 2 diabetes mellitus might influence this relationship.
Methods
Study population
A total of 341 individuals (259 patients and 82 age- and sex-matched healthy participants) were enrolled at the Diabetes Outpatient Clinic of the Department of Endocrinology and Metabolism and the Hypertension Outpatient Clinic of the Department of Internal Medicine of the University Hospital of Pisa, Italy. Group inclusion criteria were: diagnosis of essential hypertension and/or type 2 diabetes according to current guidelines [18, 19], or current treatment with BP-lowering or glucose-lowering drugs. Exclusion criteria were: chronic kidney disease (National Kidney Foundation Disease Outcomes Quality Initiative [KDOQI] stage 4 and 5), current insulin therapy, prior cardiovascular events, major co-morbidities (malignancies, chronic and acute inflammatory diseases, chronic heart failure and liver insufficiency, atrial fibrillation or frequent ectopic beats), non-cardiovascular medications interfering with vascular function (i.e. hormonal therapy, steroidal and non-steroidal anti-inflammatory drugs). Patients were divided in two groups according to the presence (n = 84) or absence (n = 175) of type 2 diabetes mellitus. All pharmacologically treated patients were on a stable therapeutic regimen for at least 3 months. The study conformed to the Declaration of Helsinki, was approved by the local Ethics Committee and all patients provided written informed consent prior to entering the study.
Experimental protocol
Participants were requested to refer to the local Diabetes Unit after an overnight fasting for collection of medical history, anthropometric variables (body weight, height, and waist circumference) as well as blood and urine samples. On the following day, BP measurement and vascular assessment (endothelial function and arterial stiffness) were determined at the Hypertension Unit. All measurements were performed in the morning after an overnight fast, in a quiet air-conditioned room (22–24°C). For the duration of the study patients were kept on their usual pharmacological treatment.
Blood pressure measurement
Brachial BP was measured with the patients resting in a supine position for at least 10 min under quiet environmental conditions. BP measurement was repeated three times at 2 min intervals by a trained physician by using an automatic oscillometric device (OMRON-705IT, Omron, Kyoto, Japan). Average BP was then calculated from the last two measurements.
Arterial stiffness
Arterial tonometry was performed according to international recommendations [20] using procedures previously described [21]. Aortic pulse wave velocity (PWV) was assessed with the same device, sequentially recording pressure waveforms at the femoral and carotid site. PWV was calculated as the ratio of the surface distance between the two recording sites (subtracting the carotid–sternal notch distance from the femoral–sternal notch distance) and wave transit time. Central BP values were obtained from radial pressure waveform analysis based on a validated transfer function (SphygmoCor, AtCor Medical, Sydney, NSW, Australia). Two consecutive measurements were recorded and averaged.
Endothelial function
Brachial artery scans were obtained by high-resolution ultrasound with a 10 MHz linear array transducer (MyLab25, ESAOTE, Florence, Italy). The endothelium-dependent response was assessed as the dilation of the brachial artery in response to increased blood flow (flow-mediated dilation; FMD) [18]. In brief, a B-mode scan of the right brachial artery was obtained in the longitudinal section between 5 cm and 10 cm above the elbow, with the probe held by a stereotactic clamp to ensure steady image recordings. A paediatric cuff was placed around the forearm just below the elbow, inflated for 5 min at 300 mmHg, and then deflated to induce reactive hyperaemia. Endothelium-independent dilation was obtained by sublingual administration of 25 μg glyceryl trinitrate (GTN). Vessel diameter was measured using a real-time computerised edge detection system. FMD and response to GTN were calculated as maximal per cent increase in diameter above baseline. The intra-observer coefficient of variation for repeated FMD measurements in the lab was 14% [18]. Arterial blood flow velocity was determined by pulsed Doppler signal at a 70° angle, with the range gate in the centre of the artery and measured at baseline and within 15 s after cuff release. This insonation angle is able to provide sufficient validity and precision of blood velocity measurement [22, 23]. Peak local shear stress was calculated according to the following equation:
assuming that blood viscosity was 0.0035 Pa × s [24].
Laboratory tests
Lipid profile, plasma glucose and HbA1c were all determined according to standard laboratory procedures. Hypercholesterolaemia and the metabolic syndrome were defined according to ATP-III criteria. High-sensitive C-reactive protein (hsCRP) was measured using a high-sensitive (low detection limit, 0.3 mg/l) immunoassay (N High Sensitivity CRP, Dade Behring, Marburg, Germany). Urinary albumin was determined using a Behring Nephelometer II analyser (Dade Behring, Milton Keynes, UK) (low detection limit 0.12 mg/dl) together with urine creatinine taken from a sample of the first urine of the morning, in order to calculate urinary albumin/creatinine ratio (UACR).
Statistical analysis
All statistical analyses were performed using NCSS 2004 (NCSS, Kaysville, UT, USA). For normally distributed data, results are expressed as mean ± SD, whereas median value and 25–75% interquartile range are used for non-normally distributed data. Differences in means among groups were analysed using ANOVA and Bonferroni post-hoc analysis for normally distributed variables, or the Kruskal–Wallis z test for non-normally distributed variables; categorical variables were analysed by χ 2 test. Analysis of covariance was used to compare aortic PWV and FMD in different subgroups, as appropriate. Spearman’s rank was used to explore correlations among variables. Multiple linear regression analysis was performed including variables with significant correlation with the dependent variable and building different models. The study was powered (90%) to detect a 1.3% difference in FMD, a 0.8 m/s difference in aortic PWV and a difference of 0.20 in the slopes of regression lines, with a type I error probability of 0.05.
Results
Clinical variables
Clinical characteristics of the study population are shown in Table 1. Diabetic and non-diabetic hypertensive patients were comparable for age, sex, duration of hypertension, percentage of smokers, renal function, and UACR. Diabetic hypertensive patients showed a higher prevalence of isolated systolic hypertension (78.8% vs 63.1%, p = 0.012) in comparison with non-diabetic hypertensive patients, in spite of similar systolic BP and lower diastolic BP. Diabetic patients also had higher BMI, waist circumference, blood glucose and HbA1c values. Owing to the higher use of statins (35% vs 6%, p < 0.001), diabetic hypertensive patients had lower total- and LDL-cholesterol, and a higher prevalence of hypercholesterolaemia (defined according to ATP-III criteria or by current lipid-lowering therapy, 82% vs 63%). HDL-cholesterol was lower and triacylglycerols higher in diabetic than in non-diabetic hypertensive patients. Diabetic hypertensive patients also showed higher levels of hsCRP. Obesity (defined as BMI ≥30 kg/m2) and the metabolic syndrome, defined according to ATP-III criteria [25], were significantly more prevalent among diabetic hypertensive patients than in normoglycaemic hypertensive patients (66% vs 18%; 91% vs 40%, respectively; p < 0.001 for both). The majority of patients in both groups were on BP-lowering treatment (65% vs 59%, p = ns). Details of cardiovascular drug therapy are shown in the electronic supplementary material (ESM Table 1).
Vascular variables
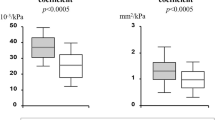
Brachial artery diameter and hyperaemic shear stress did not significantly differ between diabetic and non-diabetic hypertensive patients, although in both groups they were significantly higher than in healthy participants (Table 1). FMD was lower in non-diabetic hypertensive patients than in healthy participants, and further reduced in diabetic patients (Fig. 1). When brachial artery diameter and hyperaemic shear stress were considered as covariates, FMD was still significantly different among the three groups studied (p < 0.001). On the contrary, GTN response was not different between diabetic and non-diabetic hypertensive patients, although similarly reduced compared with healthy participants (Table 1), even after adjustment for brachial artery diameter.
PWV was higher in non-diabetic hypertensive patients than in healthy participants, and further increased in diabetic hypertensive patients (Fig. 1). The significance was not affected after considering mean BP, age and BMI as covariates (p < 0.001). Central systolic BP and pulse pressure were similarly increased in diabetic and non-diabetic hypertensive patients, while mean BP was higher in the latter group (Table 1).
Relationship between PWV and FMD: role of type 2 diabetes mellitus
In the univariate analysis performed on the whole study population, aortic PWV was significantly and negatively correlated with FMD (r = −0.272; p < 0.001). Among the other vascular variables, aortic PWV was also correlated with hyperaemic shear stress and brachial artery diameter (Table 2). However, FMD remained a significant determinant of PWV even after adjustment for these two confounders, as shown by multiple regression analysis (Model 1, Table 3). Among clinical features, PWV significantly correlated in the univariate analysis with age, brachial and central BP values, heart rate, BMI and waist circumference, cholesterol levels, triacylglycerols, fasting blood glucose and HbA1c, and hsCRP. In the multiple regression analysis including variables in Model 1 plus age, mean BP, heart rate, total and HDL-cholesterol, triacylglycerols and hsCRP, (Model 2, Table 3), FMD remained a significant predictor of PWV. After adding fasting blood glucose to the model, the relationship between PWV and FMD was no longer significant (Model 3, Table 3). In Model 3, independent predictors of PWV were: age, sex, BMI, heart rate, mean BP and fasting blood glucose (see ESM Table 2).
Univariate correlation analysis performed separately in the three groups showed that FMD was significantly correlated to PWV only in diabetic hypertensive patients, while the relationship was not significant either in healthy individuals or in normoglycaemic hypertensive patients (Table 2, Fig. 2). The slope of the relationship between FMD and PWV in diabetic hypertensive patients was steeper than in the other two groups (p < 0.05). Multivariate regression analysis performed in the diabetic group showed that FMD was an independent predictor of aortic PWV in a model adjusted for age, sex, mean BP, BMI and fasting blood glucose, (β = −0.348 [95%CI −0.577,−0.120], p = 0.003), explaining 8.3% of the variance of PWV.
Subgroup analysis in non-diabetic hypertensive patients
Among non-diabetic hypertensive patients, the relationship between FMD and PWV was investigated comparing patients with obesity (n = 33) or the metabolic syndrome (n = 69) with those without obesity and the metabolic syndrome, respectively. PWV was higher in obese hypertensive patients but not in those with the metabolic syndrome, whereas FMD was not affected by the two conditions (see ESM Table 3). FMD and PWV were not correlated in either obese or in lean hypertensive patients (r = 0.167, p = 0.360 and r = −0.118, p = 0.167). Results were also non-significant in hypertensive patients with (r = −0.006, p = 0.961) or without the metabolic syndrome (r = −0.106, p = 0.305).
The role of medications known to influence vascular function was also investigated. PWV was lower in patients on antihypertensive treatment, but was not affected by use of statins. Among BP-lowering-drug-treated patients, PWV was lower, but not significantly, in patients on renin–angiotensin system blockers. FMD was not significantly affected by any of the above-mentioned treatments (see ESM Table 3). The relationship between FMD and PWV was not influenced by the presence or absence of antihypertensive treatment (r = 0.027, p = 0.800 vs r = −0.130, p = 0.232) and by current therapy with renin–angiotensin system blockers (r = −0.029, p = 0.821 vs r = −0.110, p = 0.249) or statins (r = −0.021, p = 0.950 vs r = −0.069, p = 0.384).
Discussion
The present study explored the role of endothelial function in the peripheral conduit arteries, measured as FMD, as a predictor of arterial stiffness in hypertensive patients with and without type 2 diabetes mellitus, in the attempt to establish the role of endothelial dysfunction in the pathophysiology of arterial stiffening. The main finding of this study is that FMD is related to aortic stiffness in hypertensive patients with, but not in those without, type 2 diabetes mellitus. These results suggest that diabetes-related metabolic alterations on top of hypertension might contribute, through reduced endothelial function, to increase the wall stiffness of large arteries independently of other confounders.
An element of novelty of the present paper is the demonstration of an additive effect of hypertension and type 2 diabetes in causing an impairment of conduit artery endothelial function. Hypertensive diabetic patients had lower FMD compared with non-diabetic hypertensive patients, in the presence of similar baseline brachial artery diameter, peak shear stress and endothelium-independent dilation, highlighting a specific deleterious effect of diabetes on nitric oxide-dependent vasodilatation of conduit arteries. These results are in contrast with those obtained in small resistance arteries, showing that the concomitance of the two risk factors did not cause a further impairment of endothelial function [26, 27]. However this discrepancy should not be surprising, given the different district studied and the known heterogeneity of the vascular properties in different vascular beds [28]. On the other hand, our results confirm the presence of endothelial dysfunction and increased arterial stiffness in hypertensive patients compared with healthy participants [29, 30], as well as the further increased PWV in diabetic hypertensive patients compared with normoglycaemic hypertensive patients [17].
Brachial artery FMD and aortic PWV were negatively correlated in the whole population. This finding is in agreement with previous studies performed in mixed populations comprising healthy individuals and patients with different cardiovascular risk factors or established coronary artery disease [3, 7, 8], also including diabetic patients [4–6]. Multiple regression analysis, including vascular determinants of FMD such as baseline brachial artery diameter and hyperaemic shear stress, showed that endothelium-dependent vasodilation remained per se an independent predictor of aortic PWV. Furthermore, FMD has been shown to be a predictor of arterial stiffening independently of age and BP, which are known to be the main determinants of arterial stiffness [31], and other classical cardiovascular risk factors. Only the inclusion of fasting blood glucose caused loss of significance of the relationship between FMD and PWV in the whole population. This result suggests a specific role of glucose abnormalities in determining the association between endothelial dysfunction and increased arterial stiffness.
This hypothesis is further supported by subgroup analysis. Interestingly, FMD was a predictor of aortic PWV only among diabetic hypertensive patients. In this subgroup the relationship remained significant after taking into account age, BP values, BMI and fasting blood glucose. Thus the greater aortic stiffening found in diabetic hypertensive patients compared with patients with hypertension alone is determined, at least in part, by an independent, detrimental effect of type 2 diabetes mellitus on endothelial function. Despite the fact that the present study was not designed to investigate the pathophysiological mechanisms underlying the development of arterial damage, several factors related to type 2 diabetes mellitus may be involved, including obesity, insulin resistance and hyperinsulinaemia, low-grade inflammation, increased oxidative stress and deposition of advanced glycation end-products [14]. All these factors may concur in accelerating and worsening endothelial dysfunction, favouring arterial stiffness, although the present study suggests that neither obesity nor low-grade inflammation seem to play a major role. Moreover, we cannot rule out the possibility that, rather than a single mechanism, it is the complexity of the diabetic condition that sustains a greater and more prolonged alteration of endothelial function, leading to an increased PWV.
Because of the cross-sectional design of the study, it is not possible to establish whether the association between endothelial function and arterial stiffness in diabetes depends on common causative factors or on a cause–effect relationship. Nonetheless, growing evidence suggests that endothelial dysfunction can lead to arterial stiffness. In keeping with this hypothesis is the notion that NO, whose reduced bioavailability is the molecular basis of endothelial dysfunction, is involved in the regulation of arterial distensibility [1]. Diseases characterised by endothelial dysfunction, such as diabetes [5] and arterial hypertension [32], also show increased arterial stiffness [14, 16]. Moreover, therapeutic approaches, such as blockade of the renin–angiotensin system, improve both vascular alterations [30, 33, 34]. Conversely, the hypothesis that structural alterations associated with arterial stiffness could impair vasodilation is unlikely, since the response to GTN was not different between hypertensive patients with and without type 2 diabetes mellitus, although it was impaired compared with healthy controls. However, we cannot completely exclude the possibility that increased arterial stiffness, altering pulsatile haemodynamics, blood flow pattern and shear stress, could lead to decreased NO bioavailability [35].
In the present study, endothelial dysfunction was not a determinant of aortic stiffness either in healthy participants or in non-diabetic hypertensive patients. As far as healthy participants are concerned, our results are in agreement with a recent study carried out in a cohort of 1,754 adults, aged 30–45 years, with a very low prevalence of cardiovascular risk factors, suggesting that in young individuals PWV and FMD might reflect different aspects of cardiovascular damage [11]. In contrast McEniery et al. [10] found a significant correlation between endothelial function and PWV. This conflicting result might be attributable to biological difference of participants enrolled (wider age range and younger population compared with that in our study), as well as different methodology for the assessment of endothelial function (pulse wave analysis after albuterol inhalation, with FMD performed only in a subgroup). On the other hand, to our knowledge, this is the first study aimed, and adequately powered, to directly address the role of endothelial function in the determination of arterial stiffening in essential hypertension, since previous studies included, in pooled analysis, healthy people and did not exclude diabetic individuals [3–8]. Our results suggest that in essential hypertension the ‘functional’ component of PWV is negligible in comparison with haemodynamic load and structural wall alterations associated to BP increase, while only the presence of type 2 diabetes mellitus is able to cause endothelium-dependent stiffening of large arteries.
We also investigated the possible role of metabolic factors on top of high BP in hypertensive, normoglycaemic patients. Obesity was demonstrated to further worsen PWV but not FMD, and the relationship between the two variables was not significant either in the presence or in the absence of obesity. This observation suggests that the deleterious effects on arterial stiffness of obesity on top of hypertension are unlikely to be mediated by endothelium-related mechanisms. Furthermore, it indirectly confirms that differences found between hypertensive patients with or without type 2 diabetes mellitus were not secondary to the greater prevalence of obesity in the former group. On the other hand, our results support the hypothesis, not universally accepted in the literature, of blood pressure dependence of vascular alterations in the metabolic syndrome [36–38].
Finally, it is well known that BP-lowering and lipid-lowering treatments can affect vascular function [30, 39]. Therefore a further analysis was performed, in order to exclude the possibility that the lack of relationship between FMD and PWV in hypertensive patients could be due to the confounding effect of chronic therapies. Although chronic antihypertensive treatment was associated with a lower PWV, it did not affect the relationship between FMD and PWV.
Limitations
The cross-sectional design of the study did not allow us to determine whether the association between endothelial function and arterial stiffness that we found in diabetic patients was due to common causative factors or really reflected a cause–effect relationship. In addition, our data do not allow us to investigate the pathophysiological mechanisms underlying the development of arterial damage. Further prospective and mechanistic studies are required to confirm the present findings and to answer these crucial questions.
Some methodological limitations of our study are worth commenting on. Stimulus for FMD was evaluated as peak hyperaemic shear stress. Although this variable was chosen because a correlation with clinical risk factors was demonstrated in large cohort studies [24], emerging evidence suggests that shear rate area under the curve could be a more accurate estimation of the hyperaemic stimulus [23]. Moreover, estimation of central BP was derived by radial artery waveform through a transfer function, upon calibration with brachial BP. Although validity of transfer function in resting conditions was demonstrated in comparison with invasive central BP recordings [40], pulse pressure amplification over the brachial-to-radial arterial path can be a critical source of error [41]. Nonetheless, the method is widely accepted because it is a simple, non-invasive way to obtain a highly predictive variable in cardiovascular disease [30].
Conclusions
Our results suggest that endothelial dysfunction can contribute to arterial stiffness in diabetic hypertensive individuals independently of common confounders. Conversely, this mechanism does not seem to play a major role in patients with essential hypertension in the absence of type 2 diabetes mellitus. The greater impairment in nitric oxide availability brought about by type 2 diabetes may accelerate and worsen vessel wall distensibility, critically worsening cardiovascular prognosis inherent to this condition.
Abbreviations
- FMD:
-
Flow-mediated dilation
- GTN:
-
Glyceryl trinitrate
- hsCRP:
-
High-sensitive C-reactive protein
- PWV:
-
Pulse wave velocity
- UACR:
-
Urinary albumin/creatinine ratio
References
Wilkinson IB, Franklin SS, Cockcroft JR (2004) Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension 44:112–116
McEniery CM, Qasem A, Schmitt M, Avolio AP, Cockcroft JR, Wilkinson IB (2003) Endothelin-1 regulates arterial pulse wave velocity in vivo. J Am Coll Cardiol 42:1975–1981
Wallace SM, Yasmin McEniery CM et al (2007) Isolated systolic hypertension is characterized by increased aortic stiffness and endothelial dysfunction. Hypertension 50:228–233
Gunarathne A, Patel JV, Kausar S, Gammon B, Hughes EA, Lip GY (2009) Glycemic status underlies increased arterial stiffness and impaired endothelial function in migrant South Asian stroke survivors compared to European Caucasians: pathophysiological insights from the West Birmingham Stroke Project. Stroke 40:2298–2306
Ravikumar R, Deepa R, Shanthirani C, Mohan V (2002) Comparison of carotid intima-media thickness, arterial stiffness, and brachial artery flow mediated dilatation in diabetic and nondiabetic subjects (The Chennai Urban Population Study [CUPS-9]). Am J Cardiol 90:702–707
Jadhav UM, Kadam NN (2005) Non-invasive assessment of arterial stiffness by pulse-wave velocity correlates with endothelial dysfunction. Indian Heart J 57:226–232
Kobayashi K, Akishita M, Yu W, Hashimoto M, Ohni M, Toba K (2004) Interrelationship between non-invasive measurements of atherosclerosis: flow-mediated dilation of brachial artery, carotid intima-media thickness and pulse wave velocity. Atherosclerosis 173:13–18
Nigam A, Mitchell GF, Lambert J, Tardif JC (2003) Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. Am J Cardiol 92:395–399
Kopec G, Podolec P, Podolec J, Rubis P, Zmudka K, Tracz W (2009) Atherosclerosis progression affects the relationship between endothelial function and aortic stiffness. Atherosclerosis 204:250–254
McEniery CM, Wallace S, Mackenzie IS et al (2006) Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 48:602–608
Koivistoinen T, Virtanen M, Hutri-Kahonen N et al (2011) Arterial pulse wave velocity in relation to carotid intima-media thickness, brachial flow-mediated dilation and carotid artery distensibility: The Cardiovascular Risk in Young Finns Study and the Health 2000 Survey. Atherosclerosis 220:387–393
Wilson AM, O'Neal D, Nelson CL, Prior DL, Best JD, Jenkins AJ (2004) Comparison of arterial assessments in low and high vascular disease risk groups. Am J Hypertens 17:285–291
Lonati C, Morganti A, Comarella L, Mancia G, Zanchetti A (2008) Prevalence of type 2 diabetes among patients with hypertension under the care of 30 Italian clinics of hypertension: results of the (Iper)tensione and (dia)bete study. J Hypertens 26:1801–1808
Stehouwer CD, Henry RM, Ferreira I (2008) Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia 51:527–539
Taddei S, Virdis A, Ghiadoni L, Sudano I, Salvetti A (2001) Endothelial dysfunction in hypertension. J Cardiovasc Pharmacol 38(Suppl 2):S11–S14
Laurent S, Boutouyrie P, Asmar R et al (2001) Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37:1236–1241
Tedesco MA, Natale F, Di Salvo G, Caputo S, Capasso M, Calabro R (2004) Effects of coexisting hypertension and type II diabetes mellitus on arterial stiffness. J Hum Hypertens 18:469–473
Ghiadoni L, Penno G, Giannarelli C et al (2008) Metabolic syndrome and vascular alterations in normotensive subjects at risk of diabetes mellitus. Hypertension 51:440–445
Mancia G, de Backer G, Dominiczak A et al (2007) 2007 Guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European society of hypertension (ESH) and of the European society of cardiology (ESC). J Hypertens 25:1105–1187
Laurent S, Cockcroft J, van Bortel L et al (2006) Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27:2588–2605
Plantinga Y, Ghiadoni L, Magagna A et al (2007) Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens 20:392–397
Pyke KE, Dwyer EM, Tschakovsky ME (2004) Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol 97:499–508
Thijssen DH, Black MA, Pyke KE et al (2011) Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300:H2–H12
Mitchell GF, Parise H, Vita JA et al (2004) Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension 44:134–139
Grundy SM, Cleeman JI, Daniels SR et al (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752
Schofield I, Malik R, Izzard A, Austin C, Heagerty A (2002) Vascular structural and functional changes in type 2 diabetes mellitus: evidence for the roles of abnormal myogenic responsiveness and dyslipidemia. Circulation 106:3037–3043
Rizzoni D, Porteri E, Guelfi D et al (2001) Endothelial dysfunction in small resistance arteries of patients with non-insulin-dependent diabetes mellitus. J Hypertens 19:913–919
Deanfield J, Donald A, Ferri C et al (2005) Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens 23:7–17
Taddei S, Virdis A, Ghiadoni L, Salvetti G, Salvetti A (2000) Endothelial dysfunction in hypertension. J Nephrol 13:205–210
Ghiadoni L, Bruno RM, Stea F, Virdis A, Taddei S (2009) Central blood pressure, arterial stiffness, and wave reflection: new targets of treatment in essential hypertension. Curr Hypertens Rep 11:190–196
Cecelja M, Chowienczyk P (2009) Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension 54:1328–1336
Virdis A, Ghiadoni L, Versari D, Giannarelli C, Salvetti A, Taddei S (2008) Endothelial function assessment in complicated hypertension. Curr Pharm Des 14:1761–1770
Ghiadoni L, Magagna A, Kardasz I, Taddei S, Salvetti A (2009) Fixed dose combination of perindopril and indapamide improves peripheral vascular function in essential hypertensive patients. Am J Hypertens 22:506–512
Taddei S, Virdis A, Ghiadoni L, Sudano I, Salvetti A (2002) Effects of antihypertensive drugs on endothelial dysfunction: clinical implications. Drugs 62:265–284
Thacher T, Gambillara V, Da Silva R, Montorzi G, Stergiopulos N, Silacci P (2007) Oscillatory shear stress and reduced compliance impair vascular functions. Clin Hemorheol Microcirc 37:121–130
Czernichow S, Bertrais S, Blacher J et al (2005) Metabolic syndrome in relation to structure and function of large arteries: a predominant effect of blood pressure. A report from the SU.VI.MAX. Vascular Study. Am J Hypertens 18:1154–1160
Schillaci G, Pirro M, Vaudo G et al (2005) Metabolic syndrome is associated with aortic stiffness in untreated essential hypertension. Hypertension 45:1078–1082
Plantinga Y, Ghiadoni L, Magagna A et al (2008) Peripheral wave reflection and endothelial function in untreated essential hypertensive patients with and without the metabolic syndrome. J Hypertens 26:1216–1222
Ghiadoni L, Magagna A, Versari D et al (2003) Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension 41:1281–1286
Pauca AL, O'Rourke MF, Kon ND (2001) Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 38:932–937
Verbeke F, Segers P, Heireman S, Vanholder R, Verdonck P, van Bortel LM (2005) Noninvasive assessment of local pulse pressure: importance of brachial-to-radial pressure amplification. Hypertension 46:244–248
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
RMB recruited patients, analysed and interpreted data and drafted the article; LG and GP conceived and designed the study protocol, contributed to drafting the manuscript and revised the manuscript; GD recruited patients, analysed data and revised the manuscript; LP and DL performed laboratory exams, contributed to interpretation of data and revised the manuscript; GC, LL and FS performed vascular function tests, analysed data, and revised the manuscript; ST and SDP contributed to interpretation of data and revised the manuscript. All authors approved the final version.
Author information
Authors and Affiliations
Corresponding author
Additional information
L. Ghiadoni and S. Del Prato contributed equally to this study.
Rights and permissions
About this article
Cite this article
Bruno, R.M., Penno, G., Daniele, G. et al. Type 2 diabetes mellitus worsens arterial stiffness in hypertensive patients through endothelial dysfunction. Diabetologia 55, 1847–1855 (2012). https://doi.org/10.1007/s00125-012-2517-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-012-2517-1