Abstract
Introduction
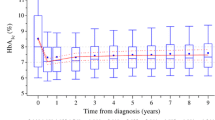
While the central role of HbA1c levels for the prediction of micro- and macrovascular complications in patients with type 1 diabetes is generally accepted; recommendations in current guidelines and the level of metabolic control actually achieved during routine care differ widely. Limited information is available on factors that influence metabolic control in the pediatric age group and during the transition from pediatric to adult diabetes care. In a large prospective multicenter database (DPV-Wiss), 338,330 individual HbA1c measurements from 27,035 patients with type-1 diabetes (94,074 observation years) were recorded between 1995 and 2005. Data were anonymously transmitted from 207 institutions. HbA1c values were mathematically standardized to the DCCT normal range (4.05–6.05%). The SAS 9.1 software was used for statistical analysis using nonparametric statistics. Median HbA1c for all measurements was 7.8%, with a strong effect of diabetes duration: median HbA1c at onset was 9.1%, during the first 2 years of diabetes 7.1% with a subsequent increase to 7.9% in patients beyond the remission phase (>2 years, 20,314 patients); a strong age dependency was present. HbA1c above the recommended guidelines was found in 23%. For all age groups, girls/women had higher HbA1c values compared to boys (mean difference 0.1%, p<0.0001). Seasonal variation was remarkably small with the lowest HbA1c values in September (mean: 7.86%) and highest values in January (8.08%; p<0.0001). Some improvement in HbA1c was observed comparing three periods: 1995–1997, 1998–2000 and 2001–2005; after remission the median HbA1c decreases from 8.5% to 7.6%. In a multivariate model, a significant influence on HbA1c was detected for age (p<0001), duration of diabetes (p<0.0001), gender (p<0.02), minority status (p<0.0001), season (p<0.0001), treatment period (p<0.0001), insulin therapy (p<0.0001) and center effect (p<0.0001).
Conclusions
Both patient-related and treatment-related variables have a strong influence on metabolic control achieved in pediatric and young adult patients with T1DM. In contrast to wide-spread belief, metabolic control is only marginally better in summer compared to winter. Some improvement in metabolic control was observed during the last 10 years.








Similar content being viewed by others
References
Amiel SA, Sherwin RS, Simonson DC et al (1986) Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med 315:215–219
Acerini CL, Cheetham TD, Edge JA (2000) Both insulin sensitivity and insulin clearance in children and young adults with type 1 (insulin-dependent) diabetes vary with growth hormone concentration and with age. Diabetologia 43:61–68
Craig ME, Handelsmann P, Donaghue KC et al; NSW/ACT HbA1c Study Group (2002) Predictors of glycemic control and hypoglycaemia in children and adolescents with type 1 diabetes from NSW and the ACT. Med J Aust 177:235–238
Diabetes Control and Complication Trial Research Group, The Effect of Intensive Treatment pof Diabetes on the developement and progression of Long-Term Complications in Insulin-Diependet Diabetes Mellitus. New Eng J of Medicine 329:977–986
Dorchy H, Roggemans MP, Willems D (1997) Glycated hemoglobin and related factors in diabetic children and adolescents under 18 years of age: a Belgian experience. Diabetes Care 20(1):2–6, Jan
Gordon CM, Mansfield JM (1996) Changing needs of the patient with diabetes mellitus during the teenage years. Curr Opin Pediatr 8:319–327
Grabert M, Schweiggert F, Holl RW (2002) A framework for diabetes documentation and quality management in Germany: 10 years of experience with DPV. Comput Methods Programs Biomed 69:115–121
Hoffmann RP, Vicini P, Sivitz WI, Cobelli C (2000) Pubertal adolescent male-female differences in insulin sensitivity and glucose effectiveness determined by the one compartement minimal model. Pediatr Res 48:384–388
Jacobson AM, Hauser ST, Willett J et al (1997) Consequences of irregular versus continuous medical follow -up in children and adolescents with insulin-dependent diabetes mellituis. J Pediatrr 131(5):727–733
Kaufman FR, Halvorson M, Carpenter S (1999) Association between diabetes control and visits to al multidisciplinary pediatric diabetes clinic. Pediatrics 03:948–951
Levine BS, Anderson BJ, Butler DA et al (2001) Predictors of glycemic control and short term adverse outcomes in youth with type 1 diabetes. J Pediatr 139(2):197–203
Mortensen HB, Hartling SG, Petersen KE (1988) A nation-wide cross sectional study of glycosylated haemoglobin in Danish children with type 1 diabetes. Diabet Med 5(9):871–876, Dec
Mortensen HB, Hougaard P (1997) Comparison of metabolic control in a cross sectional study of 2,873 children and adolescents with IDDM from 18 countries. The Hvidore study group on childhood diabetes. Diabetes Care 20(5):714–720
Mortensen HB, Robertson KJ, Aanstoot HJ et al (1998) Insulin treatment and metabolic controlof type 1 diabetes mellitus in childhood and adolescence in 18 coutries. Diab Med 15:752–759
Orchard TJ, Becker DJ, Kuller LH et al (1982) Age and sex variations in glucose tolerance and insulin responses: parallels with cardio-vascular risk. J Chronic Dis 35:123–132
Overstreet S, Holmes CS, Dunlap WP, Frentz J (1997) Sociodemographic risk factors to disease control in children with diabetes type 1. Diabet Med 14(2):153–157, Feb
Rosenbauer J, Icks A, Schmitter D, Giani G (2002) Incidence of childhood Type 1 diabetes mellitus is increasing at all ages in Germany. Diabetologia 43(3):457–458, March
Rosilio M, Cotton J-B, Wieliczko MC et al (1998) Factors assiciated with glycemic control: a cross sectional nationwide study in 2,579 French children with type 1 Diabetes. Diabetes Care 21(7):1146–1153
Sämann A, Mühlhäuser I, Bender R, Kloos Ch, Müller UA (2005) Glycaemic control and severe hypoglycaemia following training in flexible, intensive insulin therapy to enable dietary freedom in people with type 1 diabetes: a prospektive implementation study. Diabetologia 48:1965–1970
Scottish Study Group for the Care of the Young Diabetic (2001) Factors influencing glycemic control in young people with type 1 Diabetes in Scotland: a population-based study (DIABAUD 2). Diabetes Care 24(2):239–244
Urbach SL, LaFranchi S, Lambert L et al (2005) Predictors of glucose control in children and adolescents with type 1 diabetes mellitus. Ped Diab 6(2):69, Jun
Acknowledgements
This work was supported by grants from the German Ministry of Health, the German Diabetes Foundation, the Bundesärztekammer, the German Diabetes Association, the D. Buerger-Buesing Foundation and Novo Nordisk Pharma GmBH.
We are grateful to the following pediatric departments that contributed data to this study.: Uni Kinderklinik RWTH, Aachen, St. Franziskus Kinderklinik Ahlen, Zentrum Inn-Salzach, Altötting, Helius Kinderklinik Aue, Innere Augsburg, Kinderklinik Zentralklinikum Augsburg, Kinderklinik Aurich, Internist. Praxis Bad Aibling, Innere Bad Driburg/Bad Hermannsborn, Kinderklink bad hersfeld, Innere Bad Kreuznach-St. Marienwörth, Kinder-Rehaklinik Bad Kösen, Diabeteszentrum Innere bad lauterberg, Diabetesfachklinik bad Oeynhausen, Kinderklinik Lindenhof Berlin, St. Hedwig Innere Klinik Berlin, Klinik Innere Berlin Schlosspark, Kinderklinik Berlin Virchow, Innere Berlin-Hellersdorf, Kinderklinik Gilead Bielefeld, Innere Medizin Bocholt, Uni-Kinderklinik Bonn, Kinderklinik Bottrop, Knappschaftskrankenhaus Innere Bottropp, Kinderklinik Nord Bremen, Kinderklinik St. Jürgenstraße Bremen, Kinderklinik Bremerhaven, Kinderklinik Celle, Kinderklinik Chemnitz, Kinderklinik Coesfeld, Kinderklinik Prinz. Margareth,Vestische Kinderklinik Datteln, Kinderartztpraxis Deggendorf, Kinderklinik Deggendorf, Medizinische Klinik II Deggendorf, Kinderklinik Delmenhorst, Kinderklinik Detmold, Kinderklinik Dornbirn, Kinderklinik Dortmund, St. Josefsspital Innere Dortmund, Kinderklinik Uni Dresden, Birkesdorf Kinderklinik Düren, Kinderklinik Uni Düsseldorf, Innere Klinikum Barnim Werner Forßmann Ebersaldr, Kinderklinik Erfurt, Kinderklinik Uni Erlangen, Elisabeth Kinderklinik Essen, Kinderklinik Uni Essen, Städt. Kinderklinik Esslingen, Kinderklinik Eutin, St. Elisabeth Innere Eutin, Innere Uniklinik Frankfurt, Uni Kinderklinik Frankfurt, Innere Klinik Fridberg, Uni Kinderklinik Freiburg, Kinderklinik Friedrichshafen, Innere Medizin Fulda, Kinderklinik Fulda, Kinderklinik Fürth, Kinder-Fachklinik Gaißach, Kinderklinik Garmisch- Partenkirchen, Kinderklinik Geinhausen, Kinderklinik Marienhospital Gelsenkirchen, Kinderklinik Uni Gießen, Kinderklinik am Eichert, Göppingen, Städtische Kinderklinik Görlitz, Uni- Kinderklinik Göttingen, Kinderpraxis Hachenburg, Kinderklinik Hagen, Uni- Kinderklinik Halle, Städt. Kinderklinik Halle-Dölau, Altonaer Kinderklinik Hamburg, Kinderklinik Wilhelmstift Hamburg, Kinderklinik Heidberg Hamburg Nord, Kinderklinik Hamm, Kinderklinik Hanau, St. Vinzenz Innere Hanau, Innere Henriettenstift Hannover, Kinderklinik MHH Hannover, Kinderklinik auf der Bult Hannover, Uni- Kinderklinik Heidelberg, Kinderklinik Heidenheim, Innere Klinik Heilbronn, Kinderklinik Herdecke, Kinderklinik Herfordt, Inselklinik Heringsdorf, Kinderpraxis Hermeskeil, St. Elisabeth Innere Medizin Herten, Innere Kreiskrankenhaus Herzberg, Innere Hildesheim, Kinderarztpraxis Hildesheim, Kinderklinik Hildesheim, Diabetikerjugendhaus Hinrichsegen-Bruckmühl, Uni- Kinderklinik Saarland Homburg, Innere Idar Oberstein, Uni Kinderklinik Innsbruck, Kinderklinik Itzehoe, Uni- Kinderklinik Jena, Westpfalzklinikum Kinderklinik Kaiserslautern, Diabetesfachklinik Karlsburg, Städtische Kinderklinik Karlsruhe, Kinderklinik Park Schönfeld Kassel, Städt. Kinderklinik Kassel, Innere Klinik Kaufbeuren, Hl. Geis Innere, Kempen, Städt. Kinderklinik Kiel, 1. Med. Klinik Kemperhof Koblenz, Kinderklinik Kemperhof Koblenz, Innere Klinik Konstanz, Innere Klinik Krefeld, Uni Kinderklinik Köln, Kinderklinik Landshut, Uni- Kinderklinik Leipzig, Kinderklinik Leverkusen, Kinderklinik St. Bonifazius Lingen, Evangelisch Kinderklinik Lippstadt, Innere Medizin Ludwigsburg, Kinderklinik Ludwigsburg, Kinderklinik St Annastift Ludwigshafen, Uni Kinderklinik Lübeck, Uni Klinik Innere Medizin Lübeck, Kinderklinik Lüdenscheid, Uni- Kinderklinik Magdeburg, Uni- Kinderklinik Mainz, Innere Mannheim, Innere Uni Marburg, Uni Kinderklinik Marburg, Kinderklinik Mechernich, Kinderklinik Memmingen, Kinderklinik Merzig, Kinderklinik Minden Kinderklinik Moers, Kinderarztpraxis, Mutterstadt, Kinderklinik Reydth Elisabethkrankenhaus Mönchengladbach, von Haunersche Kinderklinik München, Kinderklinik München Harlaching, Kinderklinik Schwabing München, St. Franziskus Kinderklinik Münster, Uni- Kinderklinik Münster, päd. Schwerpunktpraxis Münster, Innere Kreiskrankenhaus Nagold, Kinderklinik Neuburg, Kinderklinik Kohlhof Neunkirchen, Kinderklinik Lukaskrankenhaus Neuss, Kinderklinik Elisabeth Neuwied, Cnopfsche Kinderklinik Nürnberg, Kinderklinik Klinikum Süd, Nürnberg, Innere Oberhausen, Kinderklinik Oberhausen, Kinderpraxis Bachran Oberhausen, Kinderklinik Offenbach/Main, Kinderklinik Offenburg, Kinderklinik Oldenburg, Kinderklinik Osnabrück, St. Vincenz Kinderklinik, Paderborn, Kinderarztpraxis Handwerker, Passau, Kinderklinik Passau, Pforzheim, Gemeinschaftspraxis Rastatt, Innere Kreiskrankeinhaus Rastatt, Kinderklinik St. Nikolaus Pavensburg, Dialysezentrum Innere Recklinghausen, Kinderklinik St. Hedweig, Regensburg, Kinderklinik Remscheid, Kinderklinik Rendsburg, Kinderklinik Matthiasspital, Rheine, Innere Medizin, Rosenheim, Kinderklinik Rosenheim, Uni- Kinderklinik Rostock, Universität Innere Medizin Rostock,, Kinderklinik Rotenburg/Wümme, Diabetespraxis, Saaldorf-Surheim, Kinderklinik Thüringenklinik Saalfeld, Kinderklinik Winterberg Saarbrücken, Kinderklinik Saarlouis, Reha- Kinderklinik Maximilian Scheidegg, Margaritenhospital Kinderklinik Schwäb.-Gmünd, Kinderklinik Schweinfurt, Schwerin, Kinderklinik Siegen, Hegauklinik Kinderklinik Singen, Innere Sinsheim, Innere Spaichingen, Kinderklinik Stade, Kinderklinik Stolberg, Olgahospital Kinderklinik Stuttgart, Kinderklinik Suhl, Rehaklinik Sylt, Praxis Dr. Voll, Traunstein, Kinderklinik der Borromäerinnen, Trier, Uni- Kinderklinik Tübingen, Uni- Kinderklinik Ulm, Kinderklinik Vechta, Kinderklinik Viersen, Kinderklinik Waiblingen, Kinderpraxis Biberau Waldshut, Kinderklinik Weiden, Kinderarztpraxis Weingarten, Praxis Klewer/Teichner Wetzlar, Innere Wetzlar/Braunfels, Uni- Kinderklinik Wien, Horst Schmidt- Kinderkliniken, Wiesbaden, Kinderklinik DKD Wiedbaden, St. Willehad Innere Wilhelmshafen, Kinderklinik Worms, Kinderklinik Wuppertal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerstl, EM., Rabl, W., Rosenbauer, J. et al. Metabolic control as reflectet by HbA1c in children, adolescents and young adults with type-1 diabetes mellitus: combined longitudinal analysis including 27,035 patients from 207 centers in Germany and Austria during the last decade. Eur J Pediatr 167, 447–453 (2008). https://doi.org/10.1007/s00431-007-0586-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-007-0586-9