Abstract
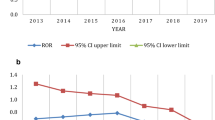
In patients with diabetes, disease per se, co-morbidities and drugs, including novel agents acting on the incretin system, have all been associated with pancreatitis with controversial data. We investigated the publicly available FDA Adverse Event Reporting System (FDA_AERS) database to gain insight into the possible association between antidiabetic agents and pancreatitis. To this aim, a case/non-case method was retrospectively performed on the FDA_AERS database (2004–2009 period). Cases were defined as reports of pancreatitis according to the Medical Dictionary for Regulatory Activities (MedDRA) terminology. All other reports associated with antidiabetics were considered non-cases. The Reporting Odds Ratio (RORs), with corresponding 95% confidential interval (CI) and Mantel–Haenszel corrected P value, was calculated as a measure of disproportionality, with subsequent time-trend analysis. We retrieved 86,938 reports related to antidiabetics, corresponding to 159,226 drug-report combinations: 2,625 cases and 156,601 non-cases. Disproportionality was found only for exenatide (number of cases, 709; ROR, 1.76; 95% CI, 1.61–1.92; P MH < 0.001) and sitagliptin (128; 1.86; 1.54–2.24; <0.001). For exenatide, significant disproportionality appeared in the first quarter of 2008 (ROR, 1.24; 95% CI, 1.10–1.40; P MH < 0.001), soon after the FDA alert; for sitagliptin in the second quarter of 2008 (1.41; 1.05–1.90; 0.021). This temporal analysis found a striking influence of relevant FDA warnings on reporting of pancreatitis (the so-called notoriety bias) and is, therefore, recommended to avoid transforming a pharmacovigilance signal of alert automatically into an alarm. The precise quantification of the risk of pancreatitis associated with antidiabetics deserves assessment through specific disease-based registries.


Similar content being viewed by others
References
Lancashire RJ, Cheng K, Langman MJ (2003) Discrepancies between population-based data and adverse reaction reports in assessing drugs as causes of acute pancreatitis. Aliment Pharmacol Ther 17:887–893
Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S (2007) Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol 5:648–661
Balani AR, Grendell JH (2008) Drug-induced pancreatitis: incidence, management and prevention. Drug Saf 31:823–837
Noel RA, Braun DK, Patterson RE, Bloomgren GL (2009) Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care 32:834–838
Gonzalez-Perez A, Schlienger RG, Rodriguez LA (2010) Acute pancreatitis in association with type 2 diabetes and antidiabetic drugs: a population-based cohort study. Diabetes Care 33:2580–2585
Girman CJ, Kou TD, Cai B, Alexander CM, O’Neill EA, Williams-Herman DE, Katz L (2010) Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes Metab 12:766–771
Garg R, Chen W, Pendergrass M (2010) Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care 33:2349–2354
Denker PS, Dimarco PE (2006) Exenatide (exendin-4)-induced pancreatitis: a case report. Diabetes Care 29:471
Tripathy NR, Basha S, Jain R, Shetty S, Ramachandran A (2008) Exenatide and acute pancreatitis. J Assoc Physicians India 56:987–988
Ahmad SR, Swann J (2008) Exenatide and rare adverse events. N Engl J Med 358:1970–1971
US Food and Drug Administration (2007) Information for healthcare professionals: exenatide (marketed as Byetta)—10/2007. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124712.htm. Accessed 6 June 2011
US Food and Drug Administration (2008) Information for healthcare professionals: exenatide (marketed as Byetta)—8/2008 Update. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm079781.htm. Accessed 6 June 2011
US Food and Drug Administration (2009) Information for healthcare professionals—acute pancreatitis and sitagliptin (marketed as Januvia and Janumet). http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHealthcareProfessionals/ucm183764.htm. Accessed 6 June 2011
Dore DD, Seeger JD, Arnold CK (2009) Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin 25:1019–1027
Engel SS, Williams-Herman DE, Golm GT, Clay RJ, Machotka SV, Kaufman KD, Goldstein BJ (2010) Sitagliptin: review of preclinical and clinical data regarding incidence of pancreatitis. Int J Clin Pract 64:984–990
Dore DD, Bloomgren GL, Wenten M, Hoffman C, Clifford CR, Quinn SG, Braun DK, Noel RA, Seeger JD (2011) A cohort study of acute pancreatitis in relation to exenatide use. Diabetes Obes Metab 13:559–566
Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC (2011) Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 141:150–156
Hawkes N (2011) Journal withdraws article after complaints from drug manufacturers. BMJ 342:d2335
BMJ News (2011) Replies to “Journal withdraws article after complaints from drug manufacturers”. http://www.bmj.com/content/342/bmj.d2335.full/reply#bmj_el_256671. Accessed 6 June 2011
European Association for the Study of Diabetes (2011) EASD commentary on the publication by Elashoff et al., published online in Gastroenterology, February 2011: Increased incidence of pancreatitis and cancer among patients given glucagon like peptide-1-based therapy. http://www.easd.org/easdwebfiles/statements/Elashoff_Commentary.pdf. Accessed 6 June 2011
US Food and Drug Administration (2011) The adverse event reporting system (AERS): latest quarterly data files. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/ucm082193. Accessed 6 June 2011
US Food and Drug Administration (2011) Drugs@FDA data files. http://www.fda.gov/Drugs/InformationOnDrugs/ucm079750.htm. Accessed 6 June 2011
EUROMEDSTAT (2007) A project funded by the European Union Commission. http://www.euromedstat.cnr.it/. Accessed 6 June 2011
WHO Collaborating Centre for Drug Statistics Methodology (2010) ATC/DDD Index 2011. http://www.whocc.no/atc_ddd_index/. Accessed 6 June 2011
Poluzzi E, Raschi E, Motola D, Moretti U, De Ponti F (2010) Antimicrobials and the risk of torsades de pointes: the contribution from data mining of the US FDA adverse event reporting system. Drug Saf 33:303–314
Bate A, Evans SJ (2009) Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf 18:427–436
van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC (2002) A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf 11:3–10
De Bruin ML, van Puijenbroek EP, Egberts AC, Hoes AW, Leufkens HG (2002) Non-sedating antihistamine drugs and cardiac arrhythmias—biased risk estimates from spontaneous reporting systems? Br J Clin Pharmacol 53:370–374
Pariente A, Gregoire F, Fourrier-Reglat A, Haramburu F, Moore N (2007) Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: the notoriety bias. Drug Saf 30:891–898
Petri H, Urquhart J (1991) Channeling bias in the interpretation of drug effects. Stat Med 10:577–581
Hauben M, Reich L, DeMicco J, Kim K (2007) ‘Extreme duplication’ in the US FDA adverse events reporting system database. Drug Saf 30:551–554
Piccinni C, Motola D, Marchesini G, Poluzzi E (2011) Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care 34:1369–1371
Montastruc JL, Sommet A, Bagheri H, Lapeyre-Mestre M (2011) Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a Pharmaco Vigilance database. Br J Clin Pharmacol. doi:10.1111/j.1365-2125.2011.04037.x
Hauben M, Hochberg A (2008) The importance of reporting negative findings in data mining. The example of exenatide and pancreatitis. Pharm Med 22:215–219
Hartnell NR, Wilson JP (2004) Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy 24:743–749
Wang HW, Hochberg AM, Pearson RK, Hauben M (2010) An experimental investigation of masking in the US FDA adverse event reporting system database. Drug Saf 33:1117–1133
Amylin Pharmaceuticals Inc (2006) New or modified indication for exenatide. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2006/021773s002,s006ltr.pdf. Accessed 6 June 2011
Begaud B, Moride Y, Tubert-Bitter P, Chaslerie A, Haramburu F (1994) False-positives in spontaneous reporting: should we worry about them? Br J Clin Pharmacol 38:401–404
Willemen MJ, Mantel-Teeuwisse AK, Straus SM, Meyboom RH, Egberts TC, Leufkens HG (2011) Use of dipeptidyl peptidase-4 inhibitors and the reporting of infections: a disproportionality analysis in the World Health Organization VigiBase. Diabetes Care 34:369–374
Mini-Sentinel (2011) Health outcomes details evidence review—pancreatitis. http://www.minisentinel.org/foundational_activities/related_projects/details.aspx?ID=125. Accessed 10 June 2011
AIFA—Italian Medicines Agency (2011) Italian antidiabetic registry. http://antidiabetici.agenziafarmaco.it/. Accessed 10 June 2011
Lee PH, Stockton MD, Franks AS (2011) Acute pancreatitis associated with liraglutide. Ann Pharmacother 45:e22
Girgis CM, Champion BL (2011) Vildagliptin-induced acute pancreatitis. Endocr Pract 17:e48–e50
Acknowledgments
The study is supported by institutional grants of the University of Bologna. G. M. received honoraries and grants from Sanofi-Aventis, Novo, Merck, Lilly. The authors thank Ariola Koci (Statistician, Department of Pharmacology, University of Bologna) for technical support in data processing.
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Raschi and C. Piccinni equally contributed to the work.
Rights and permissions
About this article
Cite this article
Raschi, E., Piccinni, C., Poluzzi, E. et al. The association of pancreatitis with antidiabetic drug use: gaining insight through the FDA pharmacovigilance database. Acta Diabetol 50, 569–577 (2013). https://doi.org/10.1007/s00592-011-0340-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-011-0340-7