Abstract
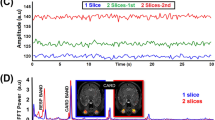
High-resolution magnetic resonance imaging (MRI) provides non-invasive images of retinal anatomy, physiology, and function with depth-resolved laminar resolution. Eye movement and drift, however, could limit high spatial resolution imaging, and anesthetics that minimize eye movement could significantly attenuate retinal function. The aim of this study was to determine the optimal anesthetic preparations to minimize eye movement and maximize visual-evoked retinal response in rats. Eye movements were examined by imaging of the cornea with a charge-coupled device (CCD) camera under isoflurane, urethane, ketamine/xylazine, and propofol anesthesia at typical dosages in rats. Combination of the paralytic pancuronium bromide with isoflurane or ketamine/xylazine anesthesia was also examined for the eye movement studies. Visual-evoked retinal responses were evaluated using full-field electroretinography (ERG) under isoflurane, ketamine/xylazine, urethane, and ketamine/xylazine + pancuronium anesthesia in rats. The degree of eye movement, measured as displacement per unit time, was the smallest under 1% isoflurane + pancuronium anesthesia. The ketamine/xylazine groups showed larger dark-adapted ERG a- and b-waves than other anesthetics tested. The isoflurane group showed the shortest b-wave implicit times. Photopic ERGs in the ketamine/xylazine groups showed the largest b-waves with the isoflurane group showing slightly shorter implicit times at the higher flash intensities. Oscillatory potentials revealed an early peak in the isoflurane group compared with ketamine/xylazine and urethane groups. Pancuronium did not affect the a- and b-wave, but did increase oscillatory potential amplitudes. Compared with the other anesthetics tested here, ketamine/xylazine + pancuronium was the best combination to minimize eye movement and maximize retinal function. These findings should set the stage for further development and application of high-resolution functional imaging techniques, such as MRI, to study retinal anatomy, physiology, and function in anesthetized rats.







Similar content being viewed by others
References
Granit R (1933) The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. J Physiol 77(3):207–239
Raitta C, Karhunen U, Seppalainen AM, Naukkarinen M (1979) Changes in the electroretinogram and visual evoked potentials during general anaesthesia. Albrecht Von Graefes Arch Klin Exp Ophthalmol 211(2):139–144
Tashiro C, Muranishi R, Gomyo I, Mashimo T, Tomi K, Yoshiya I (1986) Electroretinogram as a possible monitor of anesthetic depth. Graefes Arch Clin Exp Ophthalmol 224(5):473–476
Lin SL, Shiu WC, Liu PC, Cheng FP, Lin YC, Wang WS (2009) The effects of different anesthetic agents on short electroretinography protocol in dogs. J Vet Med Sci 71(6):763–768
Tremblay F, Parkinson JE (2003) Alteration of electroretinographic recordings when performed under sedation or halogenate anesthesia in a pediatric population. Doc Ophthalmol 107(3):271–279
Nair G, Duong TQ (2004) Echo-planar BOLD fMRI of mice on a narrow-bore 9.4 T magnet. Magn Reson Med 52(2):430–434
Sandalon S, Ofri R (2009) The effect of topical anesthesia on the rat electroretinogram. Doc Ophthalmol 118(2):101–108
Gao F, Mapleson WW, Vickers MD (1991) Effect of sub-anaesthetic infusions of propofol on peak velocity of saccadic eye movements. Eur J Anaesthesiol 8(4):267–276
Gao F, Marshall RW, Vickers MD (1991) Effect of low concentrations of nitrous oxide and isoflurane on peak velocity of saccadic eye movements. Br J Anaesth 66(2):179–184
Aschoff JC (1968) The effect of diazepam (Valium) on the saccadic eye movements in man. Arch Psychiatr Nervenkr 211(4):325–332
Meier RK, Dieringer N (1993) The role of compensatory eye and head movements in the rat for image stabilization and gaze orientation. Exp Brain Res 96(1):54–64
Dellepiane M, Mora R, Salami A (2007) Vestibular and optokinetic nystagmus in ketamine-anesthetized rabbits. Int Tinnitus J 13(1):15–20
Leopold DA, Plettenberg HK, Logothetis NK (2002) Visual processing in the ketamine-anesthetized monkey. Optokinetic and blood oxygenation level-dependent responses. Exp Brain Res 143(3):359–372
Laborit G, Angiboust R, Papin JP (1977) A study of eye movement for assessing recovery from anaesthesia. Br J Anaesth 49(8):805–810
Power C, Crowe C, Higgins P, Moriarty DC (1998) Anaesthetic depth at induction. An evaluation using clinical eye signs and EEG polysomnography. Anaesthesia 53(8):736–743
Yoshizumi J, Marshall RW, Vickers MD (1991) Effects of low concentrations of cyclopropane and halothane on peak velocity of saccadic eye movements. Br J Anaesth 67(6):735–740
Brown CH, Green DG (1984) Rod saturation in b-wave of the rat electroretinogram under two different anesthetics. Vision Res 24(1):87–90
Tanskanen P, Kylma T, Kommonen B, Karhunen U (1996) Propofol influences the electroretinogram to a lesser degree than thiopentone. Acta Anaesthesiol Scand 40(4):480–485
Chaudhary V, Hansen R, Lindgren H, Fulton A (2003) Effects of telazol and nembutal on retinal responses. Doc Ophthalmol 107(1):45–51
Takata I, Adachi E, Chiba J (1982) Influence of fluothane anesthesia on the human ERG. Nippon Ganka Gakkai Zasshi 86(12):2166–2171
Grinvald A, Frostig RD, Lieke E, Hildesheim R (1988) Optical imaging of neuronal activity. Physiol Rev 68(4):1285–1366
Tsunoda K, Oguchi Y, Hanazono G, Tanifuji M (2004) Mapping cone- and rod-induced retinal responsiveness in macaque retina by optical imaging. Invest Ophthalmol Vis Sci 45(10):3820–3826
Abramoff MD, Kwon YH, Ts’o D, Soliz P, Zimmerman B, Pokorny J, Kardon R (2006) Visual stimulus-induced changes in human near-infrared fundus reflectance. Invest Ophthalmol Vis Sci 47(2):715–721
Jonnal RS, Rha J, Zhang Y, Cense B, Gao W, Miller DT (2007) In vivo functional imaging of human cone photoreceptors. Opt Express 15(24):16141–16160
Duong TQ, Ngan SC, Ugurbil K, Kim SG (2002) Functional magnetic resonance imaging of the retina. Invest Ophthalmol Vis Sci 43(4):1176–1181
Cheng H, Nair G, Walker TA, Kim MK, Pardue MT, Thule PM, Olson DE, Duong TQ (2006) Structural and functional MRI reveals multiple retinal layers. Proc Natl Acad Sci USA 103(46):17525–17530
De La Garza B, Li G, Muir E, Shih YY, Duong TQ (2011) BOLD fMRI of visual stimulation in the rat retina at 11.7 Tesla. NMR Biomed 24:188–193
Shih YY, De La Garza BH, Muir ER, Rogers WE, Harrison JM, Kiel JW, Duong TQ (2011, in press) Lamina-specific functional MRI of retinal and choroidal responses to visual stimuli. Invest Ophthalmol Vis Sci
Zhang Y, Peng Q, Kiel JW, Rosende CA, Duong TQ (2011) Magnetic resonance imaging of vascular oxygenation changes during hyperoxia and carbogen challenges in the human retina. Invest Ophthalmol Vis Sci 52(1):286–291
Nair G, Tanaka Y, Kim M, Olson DE, Thule PM, Pardue MT, Duong TQ (2011) MRI reveals differential regulation of retinal and choroidal blood volumes in rat retina. Neuroimage 54:1063–1069
Muir ER, Duong TQ (2011) MRI of retinal and choroid blood flow with laminar resolution. NMR Biomed 24:216–223
Li Y, Cheng H, Duong TQ (2008) Blood-flow magnetic resonance imaging of the retina. Neuroimage 39:1744–1751
Li Y, Cheng H, Shen Q, Kim M, Thule PM, Olson DE, Pardue MT, Duong TQ (2009) Blood-flow magnetic resonance imaging of retinal degeneration. Invest Ophthalmol Vis Sci 50:1824–1830
Peng Q, Zhang Y, Oscar San Emeterio Nateras O, van Osch MJP, Duong TQ (2010, in press) Magnetic resonance imaging of blood flow of the human retina. Magn Reson Med
Bizheva K, Pflug R, Hermann B, Povazay B, Sattmann H, Qiu P, Anger E, Reitsamer H, Popov S, Taylor JR, Unterhuber A, Ahnelt P, Drexler W (2006) Optophysiology: depth-resolved probing of retinal physiology with functional ultrahigh-resolution optical coherence tomography. Proc Natl Acad Sci USA 103(13):5066–5071
Yao XC, Yamauchi A, Perry B, George JS (2005) Rapid optical coherence tomography and recording functional scattering changes from activated frog retina. Appl Opt 44(11):2019–2023
Srinivasan VJ, Chen Y, Duker JS, Fujimoto JG (2009) In vivo functional imaging of intrinsic scattering changes in the human retina with high-speed ultrahigh resolution OCT. Opt Express 17(5):3861–3877
Pardue MT, Phillips MJ, Yin H, Sippy BD, Webb-Wood S, Chow AY, Ball SL (2005) Neuroprotective effect of subretinal implants in the RCS rat. Invest Ophthalmol Vis Sci 46(2):674–682
Pardue MT, Phillips MJ, Yin H, Fernandes A, Cheng Y, Chow AY, Ball SL (2005) Possible sources of neuroprotection following subretinal silicon chip implantation in RCS rats. J Neural Eng 2(1):39–47
Yoshizumi J, Marshall RW, Sanders LD, Vickers MD (1993) Effects of small concentrations of isoflurane on some psychometric measurements. Br J Anaesth 71(6):839–844
Wade JG, Stevens WC (1981) Isoflurane: an anesthetic for the eighties? Anesth Analg 60(9):666–682
Woodward WR, Choi D, Grose J, Malmin B, Hurst S, Pang J, Weleber RG, Pillers DA (2007) Isoflurane is an effective alternative to ketamine/xylazine/acepromazine as an anesthetic agent for the mouse electroretinogram. Doc Ophthalmol 115(3):187–201
Jonsson Fagerlund M, Dabrowski M, Eriksson LI (2009) Pharmacological characteristics of the inhibition of nondepolarizing neuromuscular blocking agents at human adult muscle nicotinic acetylcholine receptor. Anesthesiology 110(6):1244–1252
Glover GH, Li TQ, Ress D (2000) Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44(1):162–167
Jinks SL, Dominguez CL, Antognini JF (2005) Drastic decrease in isoflurane minimum alveolar concentration and limb movement forces after thoracic spinal cooling and chronic spinal transection in rats. Anesthesiology 102(3):624–632
Vahle-Hinz C, Detsch O, Hackner C, Kochs E (2007) Corresponding minimum alveolar concentrations of isoflurane and isoflurane/nitrous oxide have divergent effects on thalamic nociceptive signalling. Br J Anaesth 98(2):228–235
Wixson S, Smiler I (1997) Anesthesia and Analgesia in Laboratory Animals. Academic Press, NY
Sanders RD, Patel N, Hossain M, Ma D, Maze M (2005) Isoflurane exerts antinociceptive and hypnotic properties at all ages in Fischer rats. Br J Anaesth 95(3):393–399
Jevtovic-Todorovic V, Benshoff N, Olney JW (2000) Ketamine potentiates cerebrocortical damage induced by the common anaesthetic agent nitrous oxide in adult rats. Br J Pharmacol 130(7):1692–1698
Pekoe GM, Smith DJ (1982) The involvement of opiate and monoaminergic neuronal systems in the analgesic effects of ketamine. Pain 12(1):57–73
Peduto VA, Concas A, Santoro G, Biggio G, Gessa GL (1991) Biochemical and electrophysiologic evidence that propofol enhances GABAergic transmission in the rat brain. Anesthesiology 75(6):1000–1009
Flecknell P (1987) Laboratory Animal Anesthesia. Accademic Press, London
Penn RD, Hagins WA (1969) Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature 223(5202):201–204
Pugh EN Jr, Falsini B, Lyubarsky AL (1998) The origins of the major rod-and cone- driven components of the rodent electroretinogram and the effect of age and light-rearing history on the magnitude of these components. In: TA Williams TP (ed) Photostasis and related phenomena. Plenum Press, New York, pp 93–128
Hood DC, Birch DG (1990) A quantitative measure of the electrical activity of human rod photoreceptors using electroretinography. Vis Neurosci 5(4):379–387
Robson JG, Frishman LJ (1998) Dissecting the dark-adapted electroretinogram. Doc Ophthalmol 95(3–4):187–215
Robson JG, Frishman LJ (1995) Response linearity and kinetics of the cat retina: the bipolar cell component of the dark-adapted electroretinogram. Vis Neurosci 12(5):837–850
Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA (2000) Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci 20(15):5733–5740
Maggi CA, Meli A (1986) Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: general considerations. Experientia 42(2):109–114
Maggi CA, Meli A (1986) Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 2: cardiovascular system. Experientia 42(3):292–297
Wachtmeister L (1998) Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res 17(4):485–521
Wenningmann I, Dilger JP (2001) The kinetics of inhibition of nicotinic acetylcholine receptors by (+)-tubocurarine and pancuronium. Mol Pharmacol 60(4):790–796
Mojumder DK, Wensel TG (2010) Topical mydriatics affect light-evoked retinal responses in anesthetized mice. Invest Ophthalmol Vis Sci 51(1):567–576
Duong TQ, Muir ER (2009) Magnetic resonance imaging of the retina. Jpn J Ophthalmol 53(4):352–367
Duong TQ, Pardue MT, Thule PM, Olson DE, Cheng H, Nair G, Li Y, Kim M, Zhang X, Shen Q (2008) Layer-specific anatomical, physiological and functional MRI of the retina. NMR Biomed 21(9):978–996
TQ Duong (2011) Magnetic resonance imaging of the retina: a brief historical and future perspective. Saudi J Ophthalmology 25:137–143
Acknowledgments
This work was supported by the NIH/NEI (R01 EY014211 and EY018855 to TQD) and MERIT awards from the Department of Veterans Affairs (TQD, MTP, DEO). We also thank Research to Prevent Blindness; NEI (P30EY006360); and Rehabilitation, Research and Development Service, Department of Veterans Affairs for their supports.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Govind Nair and Moon Kim contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Nair, G., Kim, M., Nagaoka, T. et al. Effects of common anesthetics on eye movement and electroretinogram. Doc Ophthalmol 122, 163–176 (2011). https://doi.org/10.1007/s10633-011-9271-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-011-9271-4