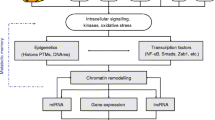
Abstract
A strong case for the deregulation of epigenetic chromatin modifications in the development and progression of various chronic complications of diabetes has emerged from recent experimental observations. Clinical trials of type 1 and type 2 diabetes patients highlight the importance of early and intensive treatment and the prolonged damage of hyperglycemia on organs such as the kidney. The functional relationship between the regulation of chromatin architecture and persistent gene expression changes conferred by prior hyperglycemia represents an important avenue of investigation for explaining diabetic nephropathy. While several studies implicate epigenetic changes at the chromatin template in the deregulated gene expression associated with diabetic nephropathy, the molecular determinants of metabolic memory in renal cells remain poorly understood. There is now strong evidence from experimental animals and cell culture of persistent glucose-driven changes in vascular endothelial gene expression that may also have relevance for the microvasculature of the kidney. Exploration of epigenetic mechanisms underlying the hyperglycemic cue mediating persistent transcriptional changes in renal cells holds novel therapeutic potential for diabetic nephropathy.

Similar content being viewed by others
References
Recently published papers of particular interest are highlighted as: • Of importance •• Of major importance
Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–88.
Campos C. Chronic hyperglycemia and glucose toxicity: pathology and clinical sequelae. Postgrad Med. 2012;124:90–7.
Ayodele OE, Alebiosu CO, Salako BL. Diabetic nephropathy–a review of the natural history, burden, risk factors and treatment. J Natl Med Assoc. 2004;96:1445–54.
Forbes JM, Fukami K, Cooper ME. Diabetic nephropathy: where hemodynamics meets metabolism. Exp Clin Endocrinol Diabetes. 2007;115:69–84.
Rhee I, Bachman KE, Park BH, et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552–6.
Laird PW, Jaenisch R. The role of DNA methylation in cancer genetic and epigenetics. Annu Rev Genet. 1996;30:441–64.
Herman JG, Baylin SB. Promoter-region hypermethylation and gene silencing in human cancer. Curr Top Microbiol Immunol. 2000;249:35–54.
Balgkouranidou I, Liloglou T, Lianidou ES. Lung cancer epigenetics: emerging biomarkers. Biomark Med. 2013;7:49–58.
Waldmann T, Schneider R. Targeting histone modifications-epigenetics in cancer. Curr Opin Cell Biol. 2013.
Webster AL, Yan MS, Marsden PA. Epigenetics and cardiovascular disease. Can J Cardiol. 2013;29:46–57.
Chin L, Gray JW. Translating insights from the cancer genome into clinical practice. Nature. 2008;452:553–63.
• Cooper ME, El-Osta A. Epigenetics: mechanisms and implications for diabetic complications. Circ Res. 2010;107:1403–13. Excellent review of the potential role of epigenetic mechansims and pathways implicated in diabetic vascular complications with insight into hyperglycemic memory.
[no authors listed] The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. The DCCT Research Group. Diabetes 1986: 35: 530–545.
[no authors listed] The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993: 329: 977–986.
[no authors listed] Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999: 22: 99–111.
[no authors listed] Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med 2000: 342: 381–389.
[no authors listed] Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003: 290: 2159–2167.
Engerman RL, Kern TS. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987;36:808–12.
Hammes HP, Klinzing I, Wiegand S, et al. Islet transplantation inhibits diabetic retinopathy in the sucrose-fed diabetic Cohen rat. Invest Ophthalmol Vis Sci. 1993;34:2092–6.
El-Osta A, Brasacchio D, Yao D, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409–17.
•• Brasacchio D, Okabe J, Tikellis C, et al. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes. 2009;58:1229–36. This important study demonstrates that transcriptional activation of RELA is linked with persistent chromatin modifications that are regulated by histone modifying enzymes.
Ihnat MA, Thorpe JE, Kamat CD, et al. Reactive oxygen species mediate a cellular ‘memory’ of high glucose stress signalling. Diabetologia. 2007;50:1523–31.
Gilbertson DT, Liu J, Xue JL, et al. Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J Am Soc Nephrol. 2005;16:3736–41.
Steinke JM, Mauer M. Lessons learned from studies of the natural history of diabetic nephropathy in young type 1 diabetic patients. Pediatr Endocrinol Rev. 2008;5 Suppl 4:958–63.
Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond). 2013;124:139–52.
Henique C, Tharaux PL. Targeting signaling pathways in glomerular diseases. Curr Opin Nephrol Hypertens. 2012;21:417–27.
Mariappan MM. Signaling mechanisms in the regulation of renal matrix metabolism in diabetes. Exp Diabetes Res. 2012;201(2):749812.
Lan HY. Transforming growth factor-beta/Smad signalling in diabetic nephropathy. Clin Exp Pharmacol Physiol. 2012;39:731–8.
Gupta A, Gupta P, Biyani M. Targeted therapies in diabetic nephropathy: an update. J Nephrol. 2011;24:686–95.
Liu W, Lan T, Xie X, et al. S1P2 receptor mediates sphingosine-1-phosphate-induced fibronectin expression via MAPK signaling pathway in mesangial cells under high glucose condition. Exp Cell Res. 2012;318:936–43.
Ziyadeh FN. Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J Am Soc Nephrol. 2004;15 Suppl 1:S55–7.
Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25.
Leehey DJ, Singh AK, Alavi N, et al. Role of angiotensin II in diabetic nephropathy. Kidney Int Suppl. 2000;77:S93–8.
Kang SW, Adler SG, Lapage J, et al. p38 MAPK and MAPK kinase 3/6 mRNA and activities are increased in early diabetic glomeruli. Kidney Int. 2001;60:543–52.
Brosius 3rd FC. New insights into the mechanisms of fibrosis and sclerosis in diabetic nephropathy. Rev Endocr Metab Disord. 2008;9:245–54.
Kanwar YS, Wada J, Sun L, et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood). 2008;233:4–11.
Bondeva T, Ruster C, Franke S, et al. Advanced glycation end-products suppress neuropilin-1 expression in podocytes. Kidney Int. 2009;75:605–16.
Bondeva T, Roger T, Wolf G. Differential regulation of Toll-like receptor 4 gene expression in renal cells by angiotensin II: dependency on AP1 and PU.1 transcriptional sites. Am J Nephrol. 2007;27:308–14.
Yonemoto S, Machiguchi T, Nomura K, et al. Correlations of tissue macrophages and cytoskeletal protein expression with renal fibrosis in patients with diabetes mellitus. Clin Exp Nephrol. 2006;10:186–92.
• Zhang Q, Xiao X, Li M, et al. Gene expression profiling in glomeruli of diabetic nephropathy rat. Exp Biol Med (Maywood). 2012;237:903–11. This study examined transcriptome profiles of diabetic and control rat gloemruli. Furhermore, bioinformatic analysis revealed enrichment for pathways associated with diabetic nephropathy.
Wada J, Zhang H, Tsuchiyama Y, et al. Gene expression profile in streptozotocin-induced diabetic mice kidneys undergoing glomerulosclerosis. Kidney Int. 2001;59:1363–73.
Brunskill EW, Potter SS. Gene expression programs of mouse endothelial cells in kidney development and disease. PLoS One. 2010;5:e12034.
• Brennan EP, Morine MJ, Walsh DW, et al. Next-generation sequencing identifies TGF-beta1-associated gene expression profiles in renal epithelial cells reiterated in human diabetic nephropathy. Biochim Biophys Acta. 2012;1822:589–99. This study describes transcriptome profiling of renal tubular epithelial cells following stimulation with TGF-β. Numerous transcriptional changes were confirmed in renal biopsies of patients with diabetic nephropathy.
Woroniecka KI, Park AS, Mohtat D, et al. Transcriptome analysis of human diabetic kidney disease. Diabetes. 2011;60:2354–69.
Tang W, Gao Y, Li Y, et al. Gene networks implicated in diabetic kidney disease. Eur Rev Med Pharmacol Sci. 2012;16:1967–73.
[no authors listed] Effect of 6 months of strict metabolic control on eye and kidney function in insulin-dependent diabetics with background retinopathy. Steno study group. Lancet 1982: 1: 121–124.
Feldt-Rasmussen B, Mathiesen ER, Deckert T. Effect of two years of strict metabolic control on progression of incipient nephropathy in insulin-dependent diabetes. Lancet. 1986;2:1300–4.
[no authors listed] Blood glucose control and the evolution of diabetic retinopathy and albuminuria. A preliminary multicenter trial. The Kroc Collaborative Study Group. N Engl J Med 1984: 311: 365–372.
Bending JJ, Viberti GC, Bilous RW, et al. Eight-month correction of hyperglycemia in insulin-dependent diabetes mellitus is associated with a significant and sustained reduction of urinary albumin excretion rates in patients with microalbuminuria. Diabetes. 1985;34 Suppl 3:69–73.
Dahl-Jorgensen K, Brinchmann-Hansen O, Hanssen KF, et al. Effect of near normoglycaemia for two years on progression of early diabetic retinopathy, nephropathy, and neuropathy: the Oslo study. Br Med J (Clin Res Ed). 1986;293:1195–9.
Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329:304–9.
Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–40.
Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
[no authors listed] Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998: 352: 837–853.
Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
Gaede P, Tarnow L, Vedel P, et al. Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant. 2004;19:2784–8.
Gaede P, Vedel P, Parving HH, et al. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353:617–22.
Murr R. Interplay between different epigenetic modifications and mechanisms. Adv Genet. 2010;70:101–41.
Svejstrup JQ. The RNA, polymerase II transcription cycle: cycling through chromatin. Biochim Biophys Acta. 2004;1677:64–73.
El-Osta A. Glycemic memory. Curr Opin Lipidol. 2012;23:24–9.
Reddy MA, Natarajan R. Role of epigenetic mechanisms in the vascular complications of diabetes. Subcell Biochem. 2012;61:435–54.
Fuks F, Hurd PJ, Wolf D, et al. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J Biol Chem. 2003;278:4035–40.
Meehan RR, Lewis JD, Bird AP. Characterization of MeCP2, a vertebrate DNA binding protein with affinity for methylated DNA. Nucleic Acids Res. 1992;20:5085–92.
Zhang Y, Ng HH, Erdjument-Bromage H, et al. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999;13:1924–35.
Keating ST, El-Osta A. Epigenetic changes in diabetes. Clin Genet 2013. [epub ahead of print]
Sapienza C, Lee J, Powell J, et al. DNA methylation profiling identifies epigenetic differences between diabetes patients with ESRD and diabetes patients without nephropathy. Epigenetics. 2011;6:20–8.
Bell CG, Teschendorff AE, Rakyan VK, et al. Genome-wide DNA methylation analysis for diabetic nephropathy in type 1 diabetes mellitus. BMC Med Genomics. 2010;3:33.
Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120.
Havas K, Whitehouse I, Owen-Hughes T. ATP-dependent chromatin remodeling activities. Cell Mol Life Sci. 2001;58:673–82.
Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–5.
Kassner I, Barandun M, Fey M et al. Crosstalk between SET7/9-dependent methylation and ARTD1-mediated ADP-ribosylation of histone H1.4. Epigenetics Chromatin 2013: 6: 1.
Tweedie-Cullen RY, Brunner AM, Grossmann J, et al. Identification of combinatorial patterns of post-translational modifications on individual histones in the mouse brain. PLoS One. 2012;7:e36980.
Muers M. Chromatin: a haul of new histone modifications. Nat Rev Genet. 2011;12:744.
Sayyed SG, Gaikwad AB, Lichtnekert J, et al. Progressive glomerulosclerosis in type 2 diabetes is associated with renal histone H3K9 and H3K23 acetylation, H3K4 dimethylation and phosphorylation at serine 10. Nephrol Dial Transplant. 2010;25:1811–7.
Gaikwad AB, Gupta J, Tikoo K. Epigenetic changes and alteration of Fbn1 and Col3A1 gene expression under hyperglycaemic and hyperinsulinaemic conditions. Biochem J. 2010;432:333–41.
• Sun G, Reddy MA, Yuan H, et al. Epigenetic histone methylation modulates fibrotic gene expression. J Am Soc Nephrol. 2010;21:2069–80. This important study implicates the Set7 lysine methyltransferase in mesangial gene expression in response to TGF-β and hyperglycemia. This is the first study to show that enzymes and histone modifications involved in vascular glycemic memory are responsive to glucose in renal cells.
Gotzsche O, Gundersen HJ, Osterby R. Irreversibility of glomerular basement membrane accumulation despite reversibility of renal hypertrophy with islet transplantation in early experimental diabetes. Diabetes. 1981;30:481–5.
Roy S, Sala R, Cagliero E, et al. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci U S A. 1990;87:404–8.
Fang D, Guan H, Liu J, et al. Early intensive insulin therapy attenuates the p38 pathway in the renal cortex and indices of nephropathy in diabetic rats. Endocr J. 2012;59:81–90.
Okabe J, Orlowski C, Balcerczyk A, et al. Distinguishing hyperglycemic changes by Set7 in vascular endothelial cells. Circ Res. 2012;110:1067–76.
Starkey JM, Haidacher SJ, LeJeune WS, et al. Diabetes-induced activation of canonical and noncanonical nuclear factor-kappaB pathways in renal cortex. Diabetes. 2006;55:1252–9.
Keating ST, El-Osta A. Chromatin modifications associated with diabetes. J Cardiovasc Transl Res. 2012;5:399–412.
Syreeni A, El-Osta A, Forsblom C, et al. Genetic examination of SETD7 and SUV39H1/H2 methyltransferases and the risk of diabetes complications in patients with type 1 diabetes. Diabetes. 2011;60:3073–80.
Nishikawa T, Edelstein D, Brownlee M. The missing link: a single unifying mechanism for diabetic complications. Kidney Int Suppl. 2000;77:S26–30.
• Zhong Q, Kowluru RA. Epigenetic changes in mitochondrial superoxide dismutase in the retina and the development of diabetic retinopathy. Diabetes. 2011;60:1304–13. This study reveals a strong association between mitochondrial superoxide dismutase and the development of diabetic retinopathy.
Schmid H, Boucherot A, Yasuda Y, et al. Modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes. 2006;55:2993–3003.
Villeneuve LM, Reddy MA, Lanting LL, et al. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci U S A. 2008;105:9047–52.
Hovind P, Tarnow L, Rossing K, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003;26:1258–64.
Yokoyama H, Okudaira M, Otani T, et al. Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney Int. 2000;58:302–11.
El-Osta A. Remodeling is at the heart of chromatin: the heartaches of chromatin. Epigenetics. 2011;6:884–7.
Pirola L, Balcerczyk A, Tothill RW, et al. Genome-wide analysis distinguishes hyperglycemia regulated epigenetic signatures of primary vascular cells. Genome Res. 2011;21:1601–15.
Acknowledgments
The authors acknowledge grant, scholarship, and fellowship support from the National Health and Medical Research Council (NHMRC), the Diabetes Australia Research Trust (DART), the Juvenile Diabetes Research Foundation International (JDRF), the National Heart Foundation of Australia (NHF), and the Australian Postgraduate Award (APA). A. E-O is a Senior Research Fellow supported by the NHMRC. This article was supported in part by the Victorian Government’s Operational Infrastructure Support Program.
Conflict of interest
Samuel T. Keating declares that he has no conflict of interest.
Assam El-Osta declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keating, S.T., El-Osta, A. Glycemic Memories and the Epigenetic Component of Diabetic Nephropathy. Curr Diab Rep 13, 574–581 (2013). https://doi.org/10.1007/s11892-013-0383-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-013-0383-y