Abstract
The Nucleotide-binding oligomerization domain, Leucine-rich Repeat and Pyrin domain containing (NLRP) family and corresponding inflammasomes are important intracellular sensors of microbial pathogens and stress signals that promote caspase-1-mediated release of IL-1β and IL-18. Studies using targeted disruption of NLRP1 and NLRP3 have revealed key roles for these inflammasomes in innate immunity and inflammation, as well as in autoimmune diseases, metabolic disorders, and cancers. The newly identified family members NLRP6, NLRP10, and NLRP12 are emerging as important molecules regulating gut homeostasis in mouse models, as well as being correlated to human diseases. Here, we review our current knowledge of NLRP1 and NLRP3 biology, from molecular structure, function, and proposed models of activation to associations with several human disorders. New insights into novel NLRPs that act as regulators of intestinal immunity are also discussed.
Similar content being viewed by others
Introduction
Innate immune cells employ a restricted range of germ-line-encoded receptors called pattern-recognition receptors (PRRs) to recognize microbial-associated molecular patterns (MAMPs) and host-derived damage-associated molecular patterns (DAMPs). NOD-like receptors (NLRs) are an important group of intracellular PRRs that comprises 22 family members in humans and 30 in mice due to evolutionary gene duplication. The NLR family can be further divided into 3 subfamilies based on modular structure: the NODs (NOD1-5, CIITA), the NLRPs (NLRP1-14), and the IPAF subfamily (IPAF/NLRC4, NAIP). All NLRs share a central nucleotide-binding and oligomerization domain (NACHT) followed by leucin-rich repeats (LRRs) at the C-terminus (with the exception of NLRP10 which lacks these LRRs). The NLRP family is further distinguished by the presence of a pyrin domain (PYD) at the amino-terminus, whereas the other members of the NLR family exhibit a caspase activation and recruitment domain (CARD) in the same position [1]. Uniquely among the NLRP proteins, NLRP1 also incorporates a CARD domain at the C-terminus. While the structure of the NLRP proteins has been studied extensively, their functions remain poorly understood, and reports that describe novel functions for NLRP proteins are being published with increasing frequency.
This review will focus on NLRP3, which is capable of assembling a large multi-protein platform, called the inflammasome, in order to mediate secretion of IL-1β and IL-18 to initiate the inflammatory process. We also present the role of NLRP1 and the corresponding inflammasome as a classical example of NLRP biology. Furthermore, recent reports that describe novel NLRP proteins (NLRP6, NLRP10, and NLRP12) and identify roles for NLRP6 and NLRP12 in intestinal homeostasis will also be discussed.
NLRP1 inflammasome
The NLRP1 inflammasome was the first member of the NLRP family to be discovered. Three orthologs have been identified in mice (NLRP1 a-c), while only one NLRP1 gene exists in humans. NLRP1 is generally expressed in many organs, including the heart, liver, lung, thymus, and spleen. Among immune cells, Langerhans cells and peripheral blood lymphocytes express NLRP1 at particularly high levels [2]. NLRP1 was initially identified as an inducer of apoptosis due to direct interactions with caspase-2 and -9 as well as binding to apoptotic protease activating factor 1 (APAF-1), which promoted the formation of apoptosomes [3, 4]. The NLRP1 inflammasome comprises NLRP1 protein, caspase-1, caspase-5 (in humans), and apoptosis-associated speck-like protein (ASC, known also as PYCARD) [5], which combine to activate pro-inflammatory caspases [6]. Inflammasome-activating signals induce polymerization of NLRP1, leading to the assembly of NLRP1 complexes together with the adaptor ASC via PYD–PYD and CARD–CARD interactions. Pro-caspase-1 and pro-caspase-5 bind into this complex and are rapidly activated by auto-catalytic cleavage, which subsequently processes the precursor forms of IL-1β and IL-18 into mature pro-inflammatory cytokines [6, 7]. NLRP1-mediated activation of caspase-1 can be inhibited by the binding of anti-apoptotic proteins Bcl-2 and Bcl-xL, which impede NLRP1 oligomerization and activation by impairing ATP binding [8, 9].
The activation of the NLRP1 inflammasome can be triggered by NLR-mediated recognition of a variety of MAMPs. For example, the murine NLRP1 inflammasome can be activated by lethal toxin from Bacillus anthracis, which is able to induce caspase-1-dependent pyroptotic death in macrophages and dendritic cells (DCs) [10]. Bacterial components such as muramyl dipeptide (MDP) can also promote the assembly and activation of the NLRP1 complex [6]. Although data regarding NLRP1 functions are far scarcer than those that described for NLRP3, various human diseases have been associated with mutations and polymorphisms in the NLRP1 gene, thus indicating a crucial role for NLRP1 in inflammation that is described in more detail below.
NLRP3 inflammasome
NLRP3, also known as cryopirin or NALP3, is the one of the best-characterized NLRP family members. Under resting conditions, NLRP3 is strongly expressed in myeloid immune cells, particularly splenic conventional DCs, monocytes, macrophages, and neutrophils [11]. NLRP3 expression can also be boosted by various Toll-like receptor (TLR)-ligands and inflammatory cytokines, including LPS, CpG, and TNF-α [11].
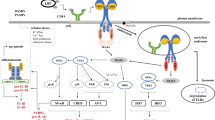
NLRP3 proteins are normally maintained in an auto-repressive state by LRR-mediated binding to chaperone proteins, such as ubiquitin ligase–associated protein SGT1 and heat shock protein 90 (HSP90) [12]. Upon activation by diverse stimuli, NLRP3 proteins polymerize and bind to the ASC adaptor, which in turn promotes the recruitment of pro-caspase-1 through CARD–CARD interactions (Fig. 1). The NLRP3 inflammasome serves to promote caspase-1-mediated activation of IL-1β and IL-18 in a way similar to the NLRP1 inflammasome, although pro-caspase-5 is not part of the NLRP3 complex.
Regulation of NLRP3 inflammasome activation. NLRP3 senses a vast array of stimuli including intact pathogens, microbial-associated molecular patterns (MAMPs), exogenous particulates, adjuvants, and damage-associated molecular patterns (DAMPs), which release NLRP3 from an auto-repressed state by dissociation from chaperone proteins, such as HSP90 and SGT1. NLRP3 is then free to recruit apoptosis-associated speck-like protein containing a CARD (ASC) and pro-caspase-1, leading to the formation of the inflammasome complex. Precursors of IL-1β and IL-18 are then released by caspase-1 cleavage. All the pathways triggering NF-κB activation are crucial to drive expression of both pro-IL-1β and IL-18 precursors, but also NLRP3. Three models of NLRP3 activation have been proposed. 1. Extracellular ATP binding to P2X7 receptor causes rapid K+ efflux, which promotes the recruitment and opening of the pannexin 1 pore. Large molecules, including MAMPs and DAMPs, may then access the cytosol through the pannexin 1 pore and potentially engage NLRP3. This cationic imbalance may also explain the NLRP3 activation that can be observed upon loss of cellular membrane integrity following exposure to pore-forming toxins. 2. Phagocytosis of insoluble particulates leads to lysosomal membrane destabilization. Lysosomal leakage results in the release of proteases, such as cathepsin B, which is known to induce NLRP3-inflammasome activation through an unknown mechanism. 3. Phagocytosis of small particulates and whole microbes provokes NADPH oxidase-dependent production of reactive oxygen species (ROS), which lead to the release of thioredoxin-interacting protein (TXNIP) from the anti-oxidant protein thioredoxin (TRX). TXNIP binding to LRR domain of NLRP3 promotes the dissociation of NLRP3-associated inhibitor complex HSP90-SGT1. Finally, a series of observations have suggested that mitochondrial ROS production might constitute the unifying mechanism of NLRP3 activation. ROS promotes mitochondrial permeability transition (MPT) to intensify further mitochondrial ROS production and NLRP3 and ASC localize to mitochondria. Inactivation of the mitochondrial voltage-dependent anion channel (VDAC) exchanging metabolites and ions abrogates caspase-1 activation. Drugs specifically potentiating ROS production by mitochondria lead to activation of NLRP3, which, mediates release of mitochondrial DNA (mtDNA). Furthermore, inhibition of autophagy/mitophagy causes NLRP3 activation
Activating signals for the NLRP3 inflammasome
Foreign “non-self” signals
Various microbial stimuli have been identified as inducers of NLRP3 inflammasome activation. For example, it was reported that Neisseria gonorrhoeae infection is able to elicit NLRP3 oligomerization, cathepsin B activation, and pro-inflammatory cytokine secretion [13]. A number of other bacterial components, including MDP, hemolysins, and toxins derived from Vibrio vulnificus and Vibrio cholerae, as well as alpha-hemolysin from Staphylococcus aureus, have all been implicated in caspase-1 activation following NLRP3 assembly [14, 15].
The NLRP3 inflammasome can also display anti-fungal activity, since NLRP3-deficient mice are hyper-susceptible to Candida albicans infection, while Aspergillus fumigatus hyphae can stimulate the NLRP3 inflammasome via the production of reactive oxygen species (ROS) [16, 17]; the important role of ROS in activating the NLRP3 inflammasome will be analyzed in more detail later. Furthermore, it has been reported that heat-killed Saccharomyces cerevisiae or purified cell wall components zymosan and mannan, when combined with ATP, can stimulate macrophages and DCs, thereby activating caspase-1 and promoting IL-1β secretion [18]. In addition, the NLRP3 inflammasome is a major player in the recognition and control of viral infections, including Influenza A virus, Sendai virus, adenovirus, vaccinia virus and encephalomyocarditis virus [19–23]. For example, double-stranded RNA (dsRNA) and viral RNA are able to induce IL-1β and IL-18 production through the activation of caspase-1 following NLRP3 activation [24]. Maturation of IL-1β precursors in macrophages can also be promoted after internalization of adenoviral DNA [25].
Host “danger” signals
NLRP3 is the only NLRP family member able to recognize host DAMPs in addition to MAMPs derived from pathogens. DAMPs are specific signals produced by cells in response to cellular damage or stress, including uric acid crystals (or monosodium urate; MSU), which are known to be the causative agent of gout [26–28]. Other crystalline particulates such as silica and asbestos can also activate the NLRP3 inflammasome to induce inflammatory disorders, such as silicosis and asbestosis. In addition, the basic calcium phosphate crystals that are associated with severe osteoarthritis and acute peri-articular inflammation also seem to play a role in NLRP3 inflammasome activation and IL-1β secretion [29]. The adjuvant properties of aluminum hydroxide (alum) have similarly been shown to depend on activation of the NLRP3 inflammasome [30, 31].
Serum amyloid A (SAA) is a crucial acute-phase protein in amyloid A-type amyloidosis that can contribute to NLRP3 activation during inflammation. SAA was reported to enhance the expression of IL-1β precursor through TLR2 and TLR4 signaling, ultimately leading to increased caspase-1-dependent IL-1β production. SAA seems to activate caspase-1 and the NLRP3 inflammasome through the P2X7 receptor and a cathepsin B-sensitive pathway [32]. In addition, fibrillar amyloid-β is involved in the pathogenesis of Alzheimer’s disease and can activate NLRP1 and NLRP3 inflammasomes, promote K+ efflux from neurons, and drive the production of IL-1β and IL-18 [33].
Lipids have also been identified as potential mediators of NLRP3 inflammasome activation. For example, cholesterol crystals are a hallmark of atherosclerotic lesions and can activate the NLRP3 inflammasome in vitro and in vivo in a similar fashion to other crystalline ligands [34, 35]. These endogenous danger signals are able to induce phago-lysosomal damage and acute inflammation. Accordingly, mice that lack NLRP3 components and cathepsin B exhibit impaired pro-inflammatory cytokine production [34, 35].
Recent papers have reported a crucial role for the NLRP3 inflammasome in several metabolic diseases, such as atherosclerosis, type 2 diabetes (T2D) and obesity [36]. In T2D and insulin resistance, elevated glucose and islet amyloid polypeptide are the main inducers of IL-1β production and NLRP3 activation in the pancreas. Together with these ligands, saturated fatty acids such as palmitate derived from high-fat diets contributed to the inflammatory process [37]. Palmitate-induced IL-1β production results in impaired insulin signaling and increased production of TNF-α [38]. Ceramide lipids have also been implicated in NLRP3 inflammasome priming and consequent caspase-1 cleavage in macrophages from obese individuals [38].
Mechanisms of inflammasome activation
Inflammasome activation is mediated by a series of cellular processes that are tightly controlled in order to prevent damaging effects to the host. A general requirement for priming before activation is one such control on NLRP3 inflammasome activity. Mouse macrophages and human monocytes stimulated with TLR ligands exhibit NF-κB-mediated increases in NLRP3 mRNA [39]. Bauernfeind and colleagues clearly showed that the NLRP3 priming requirement (as assessed by caspase-1 cleavage) was lost in wild-type cells that stably over-expressed NLRP3 [39]. These data suggest that NLRP3 expression level can modulate NLRP3 inflammasome activity in macrophages and DCs [40]. These and other observations have led to the hypothesis that activation of the NLRP3 inflammasome is a 2-step process; NF-κB-mediated transcription of NLRP3 gene licenses NLRP3 inflammasome formation, promoting ASC recruitment and caspase-1 cleavage in the presence of NLRP3 activators (Fig. 1).
Little is known about the mechanisms that drive inflammasome activation. Although activators such as MDP can bind directly to the LRR domain of NLRP1 [6, 41], it seems unlikely that a plethora of MAMPs and DAMPs with very different molecular structures could all interact directly with NLRP proteins. It seems more likely that the assembly and activation of the NLRP3 inflammasome is instead mediated by the cellular stress that induces endogenous DAMPs. Certain intracellular host ligands or mediators may be revealed only under conditions of cell stress, thus becoming available for direct or indirect activation of NLRPs. Based on the physical, chemical, and biological characteristics of the inflammasome-activating agents identified thus far, the following 3 models of inflammasome activation have been proposed (Fig. 1).
The channel model
ATP and the bacterial toxins nigericin and maitotoxin are caspase-1 activators that promote cellular potassium efflux culminating in IL-1β release [42, 43]. ATP engages the P2X7 cation selective channel, leading to pore formation and rapid potassium efflux that is counterbalanced by calcium influx. Nigericin instead acts as an ionophore that permits neutral K+/H+ exchange across membranes. The ability of ATP and nigericin to trigger both potassium efflux and activation of NLRP3 inflammasome has led to the hypothesis that intracellular decreases in potassium may act as a common trigger for NLRP1 and NLRP3 (Fig. 1). Indeed, inhibition of potassium efflux by increasing extracellular potassium levels or by blocking potassium channels with glibenclamide can prevent NLRP1 and NLRP3 activation [44, 45]. In contrast, NLRP3 activation by intracellular bacteria, such as Listeria monocytogenes and Salmonella typhimurium, does not require loss of intracellular potassium, in a way similar to the flagellin-mediated activation of the IPAF inflammasome [46].
ATP engagement of the P2X7 receptor leads to recruitment and gradual opening of pannexin 1 hemi-channels, which is associated with IL-1β release (Fig. 1) [47]. In addition to potassium efflux, the pannexin 1 pore allows passage of very large molecules between the extracellular space and cytoplasm. It has previously been reported that pannexin 1 activity is critical for caspase-1 activation induced by bacterial MAMPs and heat-killed bacteria, although only in the presence of ATP [48]. This and other observations posit the theory that the pannexin 1 pore can directly promote the delivery of bacterial ligands into the cytosol, thereby facilitating NLRP3 inflammasome activation (Fig. 1). Indeed, Marina-Garcia and colleagues demonstrated that MDP can be internalized by a mechanism requiring functional pannexin 1 to trigger caspase-1 activation in response to ATP stimulation [49]. However, the induction of pore formation by molecules derived from pathogens such as Listeria monocytogenes and Bacillus anthrax, as well as the subsequent delivery of microbial ligands that can activate caspase-1, are still poorly understood processes.
Lysosomal destabilization
Various data indicate that particulate compounds such as silica, aluminum salts, MSU crystals, and asbestos can activate NLRP3 inflammasome-dependent IL-1β release only after phagocytosis. Indeed, cytochalasin D pre-treatment of human peripheral blood mononuclear cells or murine macrophages abrogates IL-1β release upon treatment with MSU or silica [50, 51]. Once acquired, large crystals caused lysosomal acidification and leakage followed by release of lysosomal contents into the cytosol (Fig. 1) [50]. Loss of lysosomal integrity resulted in the release of lysosomal proteases, including the cysteine protease cathepsin B. Inhibition of cathepsin B activity by the pharmacological inhibitor Ca-074-Me partially decreased NLRP3 activity in LPS-primed murine macrophages stimulated with silica [50]. A similar mechanism was demonstrated in microglial cells stimulated with amyloid-β [52]. Cathepsin B inhibition impaired IL-1β release in a dose-dependent manner, whereas cathepsin L and D inhibitors had no effect [52]. However, cathepsin B-deficiency in bone marrow-derived macrophages had no effect on IL-1β secretion and caspase-1 cleavage in response to other particulates, such as hemozoin, MSU or alum [53]. These discrepancies may be due to off-target effects of the inhibitors used in these studies or perhaps indicate that other cathepsins may also be involved in inflammasome activation. The mechanism underlying the contribution of cathepsin to inflammasome activation currently remains unclear. Together with the fact that NLRP3 inflammasome activation can be triggered by artificial lysosomal damage [50], these observations suggest that the NLRP3 inflammasome senses perturbations of intracellular homeostasis rather than particulate ligands per se.
Production of reactive oxygen species
A third model proposes that the unifying activator of the NLRP3 inflammasome is the generation of ROS (Fig. 1). Normally, ROS are generated at low levels in resting cells, but concentration can rapidly increase upon cellular stress triggered by a myriad of agents (MAMPs, whole bacteria, metabolic stress, phagocytosis, cytokines, etc.). In phagocytic cells, ROS are usually formed by two major mechanisms, one of which is de novo synthesis by NADPH oxidase (in macrophages and neutrophils) or by myeloperoxidase (in neutrophils only). ROS are also an unavoidable by-product of respiration in mitochondria, due to the leakage of electrons, which can reduce oxygen molecules in the cytoplasm to form the superoxide anion. ROS are harmful for cells if not maintained at low levels, hence several defense mechanisms have evolved to control ROS-mediated damage, including the enzymes superoxide dismutase and catalase.
All known activators of the NLRP3 inflammasome induce ROS production in mouse and human macrophages and DCs [54]. Several studies have now shown that NLRP3 inflammasome activation induced by particulates [51], ATP [55], nigericin [56], and yeast [17] can be abrogated by ROS scavengers, suggesting that ROS may act upstream of NLRP3 activation. However, ROS appear to be necessary but not sufficient to mediate this process, since a number of signals which generate ROS do not activate the NLRP3 inflammasome.
When macrophages attempt to phagocytose large particulates and fibers such as MSU and asbestos (by a mechanism termed “frustrated phagocytosis”), ROS were generated by the assembly and activation of NADPH oxidase [51]. Particulate-induced activation of caspase-1 and IL-1β release is strongly impaired by pharmacological inhibitors of NADPH activity. The same effect was observed in a human monocytic cell line in which the expression of NADPH subunit p22phox was knocked down by small hairpin (sh)RNA, suggesting a crucial role for NADPH-generated ROS in inflammasome activation. However, other investigators have reported that NLRP3-mediated IL-1β is produced at normal levels by murine macrophages deficient in NADPH subunits NOX1-4 [57]. Furthermore, monocytes from chronic granulomatous disease patients characterized by defects in p22phox, p47phox, or gp91phox still activated caspase-1 and released active IL-1β normally in response to danger signals [58–60]. These discrepancies could perhaps be explained by the fact that ROS involvement in caspase-1 activation may differ between mice and humans, or they might indicate the presence of alternative pathways and functional redundancy.
Together, these data highlight the importance of using the most appropriate available models (such as genetic knockout/knockdown), rather than pharmacological inhibitors to study the contribution of ROS-mediated oxidative stress in NLRP3 inflammasome activation. Indeed, the NOX inhibitor diphenyleneiodonium chloride (DPI) and the ROS scavenger N-acetyl cysteine (NAC) have been shown to reduce the expression of NLRP3 (the priming step), rather than impairing NLRP3 activation in response to inflammatory signals, which has lead to confounding results.
Two groups have recently shown that mitochondria are indeed the main source of ROS sensed by the NLRP3 inflammasome (Fig. 1) [61, 62]. The blocking of key electron transport chain enzymes in murine macrophages and in the THP-1 monocytic cell line increased caspase-1 activation and IL-1β release [61]. A second association between NLRP3 and mitochondria comes from the observation that activated NLRP3 appears to traffic from the endoplasmic reticulum (ER) to perinuclear areas similarly to mitochondria-associated ER membranes [61].
These findings have prompted further investigation into the exact mechanism that drives ROS-mediated inflammasome activation. Thioredoxin-interacting protein (TXNIP) was recently identified as an NLRP3 binding partner [63]. In resting cells, TXNIP binds to the thioredoxin (TRX) reductase, but upon stimulation with MSU, hydrogen peroxide, or R-837, the levels of intracellular ROS increased, resulting in dissociation of the TXNIP/TRX complex and allowing the TXNIP binding to NLRP3. Although this first key link between TXNIP and NLRP3 has now been identified, further studies will be required to clarify the nature of the crosstalk between these pathways, which also bears upon inflammatory diseases in which mitochondrial dysfunction might play a role.
NLRP inflammasomes in innate and adaptive immunity
IL-1β and IL-18 are key cytokines in innate surveillance and control of invading pathogens [64]. Upon binding to their receptors, IL-1β and IL-18 trigger several signaling pathways, which lead to the transcription of pro-inflammatory genes. The subsequent up-regulation of cytokine production promotes the recruitment of inflammatory cells, as well as phagocytosis and killing of bacteria and fungi.
In addition to numerous functions in innate immunity, IL-1β and IL-18 also play prominent roles in polarizing T helper cell responses. Several studies have highlighted the important in vivo role played by NLRP3 in various mouse models of Th1/Th17-mediated autoimmune diseases, including experimental autoimmune encephalomyelitis [65]. ATP activation of the NLRP3 inflammasome in DCs is also critical for the differentiation of tumor-specific CD8+ T cell responses by promoting the production of IFN-γ [66]. Substantial data now highlight the importance of NLRP3 in driving IL-1β-mediated differentiation of Th17 cells. A recent study revealed the ability of uric acid crystals to induce potent Th17 responses in the presence of NF-κB activators [67]. These effects were dependent on the inflammasome-related cytokines IL-1α/β and IL-18 and required ASC and caspase-1. Accordingly, NLRP3 deficiency significantly impaired Th17 polarization in vitro [67]. Similarly, adenylate cyclase toxin from Bordetella pertussis was shown to activate the NLRP3 inflammasome and promoted antigen-specific Th17 responses [68]. Mice carrying the NLRP3 mutation R258W, which is associated with Muckle-Wells syndrome in humans, displayed NLRP3 hyperactivation resulting in aggressive skin inflammation dominated by Th17 cells [69].
A few recent studies have also addressed the possible role of the NLRP3 inflammasome in Th2-skewed immunity such as the one induced by alum. The production of IgE antibodies clearly depends on NLRP3, whereas a requirement for NLRP3 in IgG1 production remains uncertain [70].
Emerging family members: NLRP6, NLRP10, and NLRP12
While the pro-inflammatory roles of NLRP1 and NLRP3 have been studied extensively, the functions of alternative NLRP family members have yet to be fully uncovered. NLRP6, NLRP10, and NLRP12 are now the focus of intensive research, and our current incomplete knowledge of these genes will be presented in the next section.
NLRP6
Like many other NLRP family members, NLRP6 has undergone several changes in nomenclature over the years. Previously known as PYPAF5, this novel molecule was initially characterized as a regulator of NF-κB and caspase-1. The first published article describing PYPAF5 reported that co-expression with ASC resulted in the activation of NF-κB and induced caspase-1-dependent cytokine processing (Box 1) [69]. Another early publication focusing on the identification of murine PYPAF orthologs highlighted that PYPAF5 had been reported as an NF-κB and caspase-1 regulator as well as an angiotensin and vasopressin receptor [71]. Lech et al. [72] have described the pattern of mRNA expression for NLRP family members in human and mouse tissues, as well as in cells of the immune system in response to various stimuli. Using splenic NLRP expression as a reference, murine NLRP6 was found to be expressed at a high level in liver, kidney, and small intestine. The developmental control of NLRP6 was elucidated by Kempster et al. [73] in a rat model. Up-regulation of NLRP6 mRNA and IL-18 cytokine levels was observed during late gestation (embryonic day 20) in fetal rat intestine. Moreover, NLRP6 mRNA expression was up-regulated in a human intestinal cell line when the PPAR-γ agonist rositaglizone was added to the culture.
NLRP10
Published data describing NLRP10, also known as PYNOD, are relatively scarce compared with reports on the other NLRP family members, probably indicating a less advanced stage of research on this molecule. NLRP10 was first identified as a NLRP protein lacking the LRR at the C-terminus [74], and it was later reported that NLRP10 may exert a role as a DNA-binding protein [75]. Following the introduction of GTPase-encoding tumor suppressor gene ANXA into a prostate cancer cell line, NLRP10 was found to bind to the promoter of the 5-lipoxygenase gene. In an article focusing on a different member of the NLRP family, it was also briefly reported that NLRP10 is able to interact with the FAS-binding protein FAF-1 [76]. A more in-depth study of NLRP10 was published by Wang et al. [74] in 2004, reporting that human NLRP10 mRNA is expressed in various tissues and cell lines, with high level expression in heart, brain, and skeletal muscle. It was also shown that NLRP10 binds to ASC and can inhibit NF-κB activation and apoptosis, as well as caspase-1-mediated IL-1β maturation. A role for NLRP10 as a negative regulator of inflammation was subsequently established by Imamura et al. [77]. Macrophages and neutrophils from NLRP10 transgenic mice exhibited decreased IL-1β processing and secretion after microbial infection. However, NLRP10 expression alone was not sufficient to inhibit caspase-1 processing. Moreover, NLRP10 transgenic mice displayed better survival than wild-type mice after the injection of a lethal dose of LPS, suggesting that NLRP10 could be a negative regulator of LPS-induced endotoxic shock [77].
NLRP12
NLRP12, also known as PYPAF-7, CATERPILLAR, and Monarch-1, is perhaps the best-studied protein among the non-classical NLRP molecules. The first article to describe this protein reported an expression pattern restricted to immune cells. In terms of function, it was found that co-expression of NLRP12 and ASC resulted in the activation of both NF-κB and caspase-1, thus suggesting a role akin to NLRP3 in activating inflammation [78]. However, all subsequent references have suggested a different functionality for NLRP12 as a negative regulator of inflammation.
Williams et al. [79] reported that NLRP12 was expressed in human myeloid/monocytic cells and controlled the expression of MHC class I genes in U937 cells and that silencing NLRP12 decreased the expression of MHC class I. This apparent correlation between NLRP12 and MHC class I expression has also been highlighted in a human transplantation setting [80]. The group that first described NLRP12 in human cells also successfully demonstrated an inhibitory role for this molecule in a paper published in 2005 [81]. They showed that NLRP12 expression is down-regulated upon exposure to TNF-α and Mycobacterium tuberculosis and that NLRP12 reduces NF-κB activation mediated by MyD88, IRAK-1, and TRAF-6. In particular, they showed that NLRP12 associates with IRAK-1 upon TLR stimulation, resulting in impaired accumulation of hyperphosphorylated IRAK-1. In another series of experiments, they also determined that NLRP12 acts as a negative regulator of IL-6 expression following TLR stimulation. The conclusion of this work was that the function of NLRP12 is to dampen NF-κB activation and inhibit IL-6 cytokine expression. A subsequent report highlighted that NLRP12 is also able to inhibit CD40-mediated activation of NF-κB through the non-canonical pathway. This work, performed in a monocytic cell line, showed that NLRP12 associates with NF-κB-inducing kinase and promotes enzyme degradation by the proteasome [82]. Ye et al. [83] showed that NLRP12 binds to ATP and can hydrolyze it, which required an intact nucleotide-binding domain and conserved Walker A and B motifs. These conserved motifs are also required for self-oligomerization, association with the NF-κB-inducing kinase NIK (and its degradation) and association with IRAK (and inhibition of its phosphorylation). The results of this study suggest a model whereby ATP binding regulates the anti-inflammatory functions of NLRP12. A more recent paper has reported that NLRP12-deficient mice exhibit attenuated inflammatory responses in a mouse model of allergic dermatitis [84]. The absence of NLRP12 in DCs did not affect the expression of MHC class II or co-stimulatory molecules and did not impact on the ability of DCs to present antigen in an OT-II model. Instead, NLRP12 deficiency impaired the ability of DCs to migrate to the draining lymph nodes in vivo [84]. Moreover, NLRP12-null DCs and neutrophils failed to respond to chemokines in vitro. These findings highlight the importance of NLRP12 in maintaining both DCs and neutrophils in a migration-competent state.
NLRP6 and NLRP12 in intestinal homeostasis
The role of NLRP3 in regulating intestinal homeostasis is now well established (for a recent review, see [85]). However, the role of alternative NLRPs in gut immunity is just beginning to be elucidated, with new articles on NLRP6 and NLRP12 only appearing in the last 2 years. Chen et al. [86] reported that NLRP6-deficient mice were more susceptible to chemical colitis and colitis-induced tumorigenesis than wild-type mice. Elinav et al. [87] subsequently showed that the absence of NLRP6 in murine colonic epithelial cells resulted in reduced serum IL-18 levels. This same group also identified an important role for the NLRP6 inflammasome in maintaining the balance of the gut microbiota (Fig. 2). While the authors of this work did not identify a specific mechanism for the protective action of NLRP6 in this model beyond an involvement of IL-18, they speculated that the NLRP6 inflammasome recognizes bacterial by-products and/or general cell damage in the intestinal epithelium. This signal might then facilitate the steady-state production of IL-18. It has been shown that a decreased level of this cytokine in mice that are lacking components of the NLRP6 inflammasome plays a role in the enhanced colitogenic properties of the gut microbiota [87]. Commenting on this article, van Lookeren Campagne and Dixit highlighted that IL-18 can act in concert with IL-12 and IL-15 to promote the production of IFN-γ by NK and T helper cells [88, 89]. Since IFN-γ is able to induce marked microbicidal activity in macrophages, induction of this cytokine could be further modulating the balance of the microbiota [89]. Finally, Normand et al. [90] assessed the role of NLRP6 in tissue repair and epithelial self-renewal in mouse intestine, revealing that NLRP6-deficient mice are defective in mucosal healing compared to wild-type animals. Moreover, the absence of NLRP6 resulted in the increased expression of molecules including Csnk1ε and SMARCC1, which can enhance the proliferation of dysplastic epithelium, consistent with a previous report that NLRP6 deficiency results in increased colitis-associated tumor growth. Taken together, these data suggest a fundamental role for the NLRP6 in the maintenance of intestinal homeostasis and a healthy gut flora, as well as an important function in preventing colitis and tumorigenesis.
Scheme resuming the current knowledge of the roles of NLRP6 and NLRP12 in the gut. The epithelial cells of the intestine express both NLRP12 and NLRP6, as do myeloid-monocytic cells. Through the action of IL-18 and possibly IL-1β and other unknown mediators, they contribute to maintain intestinal homeostasis, tissue repair functionality and a non-colitogenic environment. The range of activators for both NLRP6 and NLRP12 is not fully known, but it is thought to comprise both exogenous (bacteria) and endogenous (signals of cell damage, for example) stimuli. When NLRP6 or NLRP12 are deleted via genetic knockout, gut homeostasis is impaired (deletion of NLRP6 results also in an altered gut microbiota). The tissue repair mechanisms are defective, thus leading to colitis and impaired wound healing, and tumorigenesis and general gut damage ensue
A very recent paper has highlighted for the first time that NLRP12, like NLRP6, can contribute to the maintenance of intestinal homeostasis (Fig. 2). Kanneganti et al. [91] reported that mice deficient in NLRP12 are highly susceptible to colonic inflammation and tumorigenesis; this phenotype was associated with the increased production of pro-inflammatory cytokines and chemokines. A more detailed analysis of these NLRP12-deficient mice subsequently revealed that the increased colonic inflammation and tumor risk could be attributed to a failure to down-regulate NF-κB and ERK activation in macrophages. Taken together, these publications suggest a novel role for NLRP family members in the maintenance of intestinal homeostasis.
NLRP-associated human diseases
NLRP mutations have been identified in various human pathologies. For example, several papers have reported a strong correlation between NLRP1 and NLRP3 polymorphisms with autoimmune and autoinflammatory diseases including vitiligo, type 1 diabetes and Crohn’s disease. Vitiligo is a well-known autoimmune disease characterized by depigmentation of the skin and hair due to melanocyte loss, which seems to be associated with variants in the NLRP1 gene. A susceptibility locus on chromosome 17 has now been identified in patients affected by vitiligo, and a huge number of single nucleotide polymorphisms (SNPs) were found within this gene and corresponding promoter region [92].
Another autoimmune disorder associated with polymorphisms in NLRP1 and NLRP3 genes is celiac disease, a multifactorial pathology affecting the small intestine due to a strong reaction to gluten. Mutations in NLRP1 and NLRP3 genes seem to be crucial for the development of this disease, and NLRP1 variants may correlate with increased inflammasome activation and pro-inflammatory cytokine production [93].
Polymorphisms in NLRP1 also correlate with other autoimmune pathologies such as Addison’s disease, a chronic endocrine disorder characterized by impaired adrenal gland activity. It was previously reported that certain NLRP1 variants were present in Addison’s patients that could be considered as risk factors for the onset of disease [94]. Interestingly, these same mutations also correlate well with type 1 diabetes [94]. NLRP1 was similarly identified as a susceptibility gene for the development of Alzheimer’s disease, a multifactorial disorder characterized by neuro-degeneration. In particular, four key SNPs seem to be crucial for the onset of pathology [95].
Like NLRP1, the NLRP3 inflammasome has been identified as contributing to the risk of autoimmune diseases. Gain-of-function mutations have been detected in the NLRP3 gene of patients with cryopyrin-associated periodic syndrome (CAPS) [96]. These inflammatory disorders are defined as a group of inherited autoinflammatory conditions, such as cold autoinflammatory syndrome, Muckle-Wells syndrome and neonatal onset multisystem inflammatory disease [97]. These gain-of-function mutations, primarily missense in nature, are preferentially located in exon 4 and 6, or in the promoter region [98, 99], and they induce over-production of IL-1β and the subsequent development of severe inflammation. This dysregulation of the IL-1β pathway may be due to alterations in the regulatory mechanism responsible for NLRP3 assembly and activation [97].
NLRP3 also plays a role in disorders such as systemic sclerosis, in which connective tissue becomes deeply fibrotic. The genetic profile of fibroblasts from systemic sclerosis patients has now been analyzed. Among the differentially expressed genes, NLRP3, IL-1β, and pro-caspase-1 were up-regulated, and increased expression was also observed for NLRP4 and NLRP5 [100]. It has also been reported that SNPs in the NLRP3 gene are associated with susceptibility to various viral infections, including human immunodeficiency virus (HIV). This finding is also supported by in vitro data demonstrating that HIV infection induces NLRP3 mRNA expression in human DCs from healthy donors [101]. Other viruses such as Sendai, influenza, and hepatitis C virus are also able to induce NLRP3-dependent inflammatory responses [102].
Finally, NLRP3 inflammasome activity can also influence intestinal homeostasis. Several papers have reported that dysregulation of NLRP3 inflammasome-related genes can exacerbate inflammatory bowel disease (IBD). For example, IBD is associated with SNPs in NLRP3 regulatory elements, and affected patients can exhibit extreme changes in expression of IL-1β and IL-18 genes [85]. In addition to these mutations, other SNPs have been found that correlate with the onset of disease subtypes, such as Crohn’s disease [103]. Finally, various mutations in NLRP12 have been associated with the onset of hereditary periodic fever syndromes, characterized by short attacks of fever. In these patients, increased IL-1β production and a redox state alteration have been observed [104–106].
Concluding remarks
NLRP inflammasomes mediate innate immune responses to a variety of pathogens, environmental, and endogenous signals. Despite intensive research, we have yet to fully uncover the mechanisms responsible for NLRP activation. Indeed, the three proposed models of NLRP3 activation can only partially explain the body of experimental data. Recent reports have placed NLRP3 at the center of a series of mechanisms that mediate diverse cellular functions, metabolic processes, and lineage development in addition to effects on immunological pathways. Certainly, the latest breakthrough discoveries appear to suggest close interplay between NLRP3-driven inflammation, mitochondrial dysregulation, and autophagy, not to mention the possibility that NLRP proteins might also operate independently of ASC and caspase-1. Exciting progress has recently been made in uncovering the cellular and immune functions regulated by newly discovered NLRP members, revealing a crucial role in intestinal homeostasis. These new findings will open novel avenues of investigation that will lead us to a better understanding of the inflammatory processes that cause cancers, metabolic dysfunction, obesity, and more.
References
Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003;4(2):95–104.
Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochemi Cytochem Off J Histochem Soc. 2007;55(5):443–52.
Hlaing T, Guo RF, Dilley KA, Loussia JM, Morrish TA, Shi MM, Vincenz C, Ward PA. Molecular cloning and characterization of DEFCAP-L and -S, two isoforms of a novel member of the mammalian Ced-4 family of apoptosis proteins. J Biol Chem. 2001;276(12):9230–8.
Chu ZL, Pio F, Xie Z, Welsh K, Krajewska M, Krajewski S, Godzik A, Reed JC. A novel enhancer of the Apaf1 apoptosome involved in cytochrome c-dependent caspase activation and apoptosis. J Biol Chem. 2001;276(12):9239–45.
Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–26.
Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25(5):713–24.
Nour AM, Yeung YG, Santambrogio L, Boyden ED, Stanley ER, Brojatsch J. Anthrax lethal toxin triggers the formation of a membrane-associated inflammasome complex in murine macrophages. Infect Immun. 2009;77(3):1262–71.
Faustin B, Chen Y, Zhai D, Le Negrate G, Lartigue L, Satterthwait A, Reed JC. Mechanism of Bcl-2 and Bcl-X(L) inhibition of NLRP1 inflammasome: loop domain-dependent suppression of ATP binding and oligomerization. Proc Natl Acad Sci USA. 2009;106(10):3935–40.
Bruey JM, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, Kress CL, Bailly-Maitre B, Li X, Osterman A, Matsuzawa S, Terskikh AV, Faustin B, Reed JC. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129(1):45–56.
Terra JK, Cote CK, France B, Jenkins AL, Bozue JA, Welkos SL, LeVine SM, Bradley KA. Cutting edge: resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J Immunol. 2010;184(1):17–20.
Guarda G, Zenger M, Yazdi AS, Schroder K, Ferrero I, Menu P, Tardivel A, Mattmann C, Tschopp J. Differential expression of NLRP3 among hematopoietic cells. J Immunol. 2011;186(4):2529–34.
Mayor A, Martinon F, De Smedt T, Petrilli V, Tschopp J. A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat Immunol. 2007;8(5):497–503.
Duncan JA, Gao X, Huang MT, O’Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, Ting JP. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182(10):6460–9.
Toma C, Higa N, Koizumi Y, Nakasone N, Ogura Y, McCoy AJ, Franchi L, Uematsu S, Sagara J, Taniguchi S, Tsutsui H, Akira S, Tschopp J, Nunez G, Suzuki T. Pathogenic vibrio activate NLRP3 inflammasome via cytotoxins and TLR/nucleotide-binding oligomerization domain-mediated NF-kappa B signaling. J Immunol. 2010;184(9):5287–97.
Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS ONE. 2009;4(10):e7446.
Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459(7245):433–6.
Said-Sadier N, Padilla E, Langsley G, Ojcius DM. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS ONE. 2010;5(4):e10008.
Lamkanfi M, Malireddi RK, Kanneganti TD. Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem. 2009;284(31):20574–81.
Barlan AU, Griffin TM, McGuire KA, Wiethoff CM. Adenovirus membrane penetration activates the NLRP3 inflammasome. J Virol. 2010;85(1):146–55.
Delaloye J, Roger T, Steiner-Tardivel QG, Le Roy D, Knaup Reymond M, Akira S, Petrilli V, Gomez CE, Perdiguero B, Tschopp J, Pantaleo G, Esteban M, Calandra T. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009;5(6):e1000480.
Rajan JV, Rodriguez D, Miao EA, Aderem A. The NLRP3 inflammasome detects encephalomyocarditis virus and vesicular stomatitis virus infection. J Virol. 2011;85(9):4167–72.
Thomas PG, Dash P, Aldridge JR Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30(4):566–75.
Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30(4):556–65.
Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Nunez G. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281(48):36560–8.
Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452(7183):103–7.
Shi Y, Mucsi AD, Ng G. Monosodium urate crystals in inflammation and immunity. Immunol Rev. 2010;233(1):203–17.
Ghaemi-Oskouie F, Shi Y. The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr Rheumatol Rep. 2011;13(2):160–6.
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41.
Pazar B, Ea HK, Narayan S, Kolly L, Bagnoud N, Chobaz V, Roger T, Liote F, So A, Busso N. Basic calcium phosphate crystals induce monocyte/macrophage IL-1beta secretion through the NLRP3 inflammasome in vitro. J Immunol. 2011;186(4):2495–502.
Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453(7198):1122–6.
Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181(6):3755–9.
Niemi K, Teirila L, Lappalainen J, Rajamaki K, Baumann MH, Oorni K, Wolff H, Kovanen PT, Matikainen S, Eklund KK. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol. 2011;186(11):6119–28.
Salminen A, Ojala J, Suuronen T, Kaarniranta K, Kauppinen A. Amyloid-beta oligomers set fire to inflammasomes and induce Alzheimer’s pathology. J Cell Mol Med. 2008;12(6A):2255–62.
Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE. 2010;5(7):e11765.
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–61.
De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011;32(8):373–9.
Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12(5):408–15.
Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–88.
Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183(2):787–91.
Conforti-Andreoni C, Beretta O, Licandro G, Qian HL, Urbano M, Vitulli F, Ricciardi-Castagnoli P, Mortellaro A. Synergism of NOD2 and NLRP3 activators promotes a unique transcriptional profile in murine dendritic cells. J Leukoc Biol; 2010;88(6):1207-16.
Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14(21):1929–34.
Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269(21):15195–203.
Walev I, Reske K, Palmer M, Valeva A, Bhakdi S. Potassium-inhibited processing of IL-1 beta in human monocytes. EMBO J. 1995;14(8):1607–14.
Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14(9):1583–9.
Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187(1):61–70.
Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282(26):18810–8.
Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25(21):5071–82.
Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26(4):433–43.
Marina-Garcia N, Franchi L, Kim YG, Miller D, McDonald C, Boons GJ, Nunez G. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J Immunol. 2008;180(6):4050–7.
Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9(8):847–56.
Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–7.
Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9(8):857–65.
Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, Suva ML, Stehle JC, Kopf M, Stamenkovic I, Corradin G, Tschopp J. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS ONE. 2009;4(8):e6510.
Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10(3):210–5.
Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 2008;105(26):9035–40.
Sorbara MT, Girardin SE. Mitochondrial ROS fuel the inflammasome. Cell Res. 2011;21(4):558–60.
Latz E. NOX-free inflammasome activation. Blood. 2010;116(9):1393–4.
Meissner F, Seger RA, Moshous D, Fischer A, Reichenbach J, Zychlinsky A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. 2010;116(9):1570–3.
van Bruggen R, Koker MY, Jansen M, van Houdt M, Roos D, Kuijpers TW, van den Berg TK. Human NLRP3 inflammasome activation is No1–4 independent. Blood. 2010;115(26):5398–400.
van de Veerdonk FL, Smeekens SP, Joosten LA, Kullberg BJ, Dinarello CA, van der Meer JW, Netea MG. Reactive oxygen species-independent activation of the IL-1beta inflammasome in cells from patients with chronic granulomatous disease. Proc Natl Acad Sci USA. 2010;107(7):3030–3.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–5.
Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12(3):222–30.
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2009;11(2):136–40.
Dinarello CA. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur J Immunol. 2011;41(5):1203–17.
Chen M, Wang H, Chen W, Meng G. Regulation of adaptive immunity by the NLRP3 inflammasome. Int Immunopharmacol. 2011;11(5):549-54.
Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–8.
Conforti-Andreoni C, Spreafico R, Qian HL, Riteau N, Ryffel B, Ricciardi-Castagnoli P, Mortellaro A. Uric Acid-Driven Th17 differentiation requires inflammasome-derived IL-1 and IL-18. J Immunol. 2011;187(11):5842-50.
Dunne A, Ross PJ, Pospisilova E, Masin J, Meaney A, Sutton CE, Iwakura Y, Tschopp J, Sebo P, Mills KH. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J Immunol. 2010;185(3):1711–9.
Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30(6):860–74.
Spreafico R, Ricciardi-Castagnoli P, Mortellaro A. The controversial relationship between NLRP3, alum, danger signals and the next-generation adjuvants. Eur J Immunol. 2010;40(3):638–42.
Albrecht M, Domingues FS, Schreiber S, Lengauer T. Identification of mammalian orthologs associates PYPAF5 with distinct functional roles. FEBS Lett. 2003;538(1–3):173–7.
Lech M, Avila-Ferrufino A, Skuginna V, Susanti HE, Anders HJ. Quantitative expression of RIG-like helicase, NOD-like receptor and inflammasome-related mRNAs in humans and mice. Int Immunol. 2010;22(9):717–28.
Kempster SL, Belteki G, Forhead AJ, Fowden AL, Catalano RD, Lam BY, McFarlane I, Charnock-Jones DS, Smith GC. Developmental control of the Nlrp6 inflammasome and a substrate, IL-18, in mammalian intestine. Am J Physiol Gastrointest Liver Physiol. 2011;300(2):G253–63.
Wang Y, Hasegawa M, Imamura R, Kinoshita T, Kondo C, Konaka K, Suda T. PYNOD, a novel Apaf-1/CED4-like protein is an inhibitor of ASC and caspase-1. Int Immunol. 2004;16(6):777–86.
Torosyan Y, Dobi A, Naga S, Mezhevaya K, Glasman M, Norris C, Jiang G, Mueller G, Pollard H, Srivastava M. Distinct effects of annexin A7 and p53 on arachidonate lipoxygenation in prostate cancer cells involve 5-lipoxygenase transcription. Cancer Res. 2006;66(19):9609–16.
Kinoshita T, Kondoh C, Hasegawa M, Imamura R, Suda T. Fas-associated factor 1 is a negative regulator of PYRIN-containing Apaf-1-like protein 1. Int Immunol. 2006;18(12):1701–6.
Imamura R, Wang Y, Kinoshita T, Suzuki M, Noda T, Sagara J, Taniguchi S, Okamoto H, Suda T. Anti-inflammatory activity of PYNOD and its mechanism in humans and mice. J Immunol. 2010;184(10):5874–84.
Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, Lora JM, Geddes BJ, Briskin M, DiStefano PS, Bertin J. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem. 2002;277(33):29874–80.
Williams KL, Taxman DJ, Linhoff MW, Reed W, Ting JP. Cutting edge: Monarch-1: a pyrin/nucleotide-binding domain/leucine-rich repeat protein that controls classical and nonclassical MHC class I genes. J Immunol. 2003;170(11):5354–8.
Hernández-Ruiz M, Ramírez-Alvarado A, Vela-Ojeda J, Ortiz-Navarrete V. NALP12 function as enhancer for MHC-I genes in patients undergo haematopoietic stem cell transplantation. 37th annual meeting of the European group for blood and marrow transplantation;2011.
Williams KL, Lich JD, Duncan JA, Reed W, Rallabhandi P, Moore C, Kurtz S, Coffield VM, Accavitti-Loper MA, Su L, Vogel SN, Braunstein M, Ting JP. The CATERPILLER protein monarch-1 is an antagonist of toll-like receptor-, tumor necrosis factor alpha-, and Mycobacterium tuberculosis-induced pro-inflammatory signals. J Biol Chem. 2005;280(48):39914–24.
Lich JD, Williams KL, Moore CB, Arthur JC, Davis BK, Taxman DJ, Ting JP. Monarch-1 suppresses non-canonical NF-kappaB activation and p52-dependent chemokine expression in monocytes. J Immunol. 2007;178(3):1256–60.
Ye Z, Lich JD, Moore CB, Duncan JA, Williams KL, Ting JP. ATP binding by monarch-1/NLRP12 is critical for its inhibitory function. Mol Cell Biol. 2008;28(5):1841–50.
Arthur JC, Lich JD, Ye Z, Allen IC, Gris D, Wilson JE, Schneider M, Roney KE, O’Connor BP, Moore CB, Morrison A, Sutterwala FS, Bertin J, Koller BH, Liu Z, Ting JP. Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity. J Immunol. 2010;185(8):4515–9.
Zaki MH, Lamkanfi M, Kanneganti TD. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol. 2011;32(4):171–9.
Chen GY, Liu M, Wang F, Bertin J, Nunez G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J Immunol. 2011;186(12):7187–94.
Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–57.
Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50.
van Lookeren Campagne M, Dixit VM. Immunology: in command of commensals. Nature. 2011;474(7349):42–3.
Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, Peyrin-Biroulet L, Lemoine Y, Hot D, Chamaillard M. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc Natl Acad Sci USA. 2011;108(23):9601–6.
Zaki MH, Vogel P, Malireddi RK, Body-Malapel M, Anand PK, Bertin J, Green DR, Lamkanfi M, Kanneganti TD. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20(5):649–60.
Smith AG, Sturm RA. Multiple genes and locus interactions in susceptibility to vitiligo. J Invest Dermatol. 2010;130(3):643–5.
Pontillo A, Vendramin A, Catamo E, Fabris A, Crovella S. The missense variation Q705K in CIAS1/NALP3/NLRP3 gene and an NLRP1 haplotype are associated with celiac disease. Am J Gastroenterol. 2011;106(3):539–44.
Magitta NF, Boe Wolff AS, Johansson S, Skinningsrud B, Lie BA, Myhr KM, Undlien DE, Joner G, Njolstad PR, Kvien TK, Forre O, Knappskog PM, Husebye ES. A coding polymorphism in NALP1 confers risk for autoimmune Addison’s disease and type 1 diabetes. Genes Immun. 2009;10(2):120–4.
Pontillo A, Catamo E, Arosio B, Daniela M, Crovella S. NALP1/NLRP1 genetic variants are associated with alzheimer disease. Alzheimer Dis Assoc Disord. 2011 [Epub ahead of print].
Cuisset L, Jeru I, Dumont B, Fabre A, Cochet E, Le Bozec J, Delpech M, Amselem S, Touitou I. Mutations in the autoinflammatory cryopyrin-associated periodic syndrome gene: epidemiological study and lessons from eight years of genetic analysis in France. Ann Rheum Dis. 2011;70(3):495–9.
Yu JR, Leslie KS. Cryopyrin-associated periodic syndrome: an update on diagnosis and treatment response. Curr Allergy Asthma Rep. 2011;11(1):12–20.
Jesus AA, Silva CA, Segundo GR, Aksentijevich I, Fujihira E, Watanabe M, Carneiro-Sampaio M, Duarte AJ, Oliveira JB. Phenotype-genotype analysis of cryopyrin-associated periodic syndromes (CAPS): description of a rare non-exon 3 and a novel CIAS1 missense mutation. J Clin Immunol. 2008;28(2):134–8.
Aksentijevich I, Putnam CD, Remmers EF, Mueller JL, Le J, Kolodner RD, Moak Z, Chuang M, Austin F, Goldbach-Mansky R, Hoffman HM, Kastner DL. The clinical continuum of cryopyrinopathies: novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis Rheum. 2007;56(4):1273–85.
Artlett CM, Sassi-Gaha S, Rieger JL, Boesteanu AC, Feghali-Bostwick CA, Katsikis PD. The inflammasome activating caspase 1 mediates fibrosis and myofibroblast differentiation in systemic sclerosis. Arthritis Rheum. 2011;63(11):3563–74.
Pontillo A, Silva LT, Oshiro TM, Finazzo C, Crovella S, Duarte AJ. HIV-1 induces NALP3-inflammasome expression and IL-1ss secretion in dendritic cells from healthy individuals but not from HIV+ patients. AIDS. 2012;26(1):11–8.
Burdette D, Haskett A, Presser L, McRae S, Iqbal J, Waris G. Hepatitis C virus activates interleukin-1{beta} via caspase-1-inflammasome complex. J Gen Virol. 2012;93(Pt 2):235–46.
Villani AC, Lemire M, Fortin G, Louis E, Silverberg MS, Collette C, Baba N, Libioulle C, Belaiche J, Bitton A, Gaudet D, Cohen A, Langelier D, Fortin PR, Wither JE, Sarfati M, Rutgeerts P, Rioux JD, Vermeire S, Hudson TJ, Franchimont D. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat Genet. 2009;41(1):71–6.
Jeru I, Duquesnoy P, Fernandes-Alnemri T, Cochet E, Yu JW, Lackmy-Port-Lis M, Grimprel E, Landman-Parker J, Hentgen V, Marlin S, McElreavey K, Sarkisian T, Grateau G, Alnemri ES, Amselem S. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc Natl Acad Sci USA. 2008;105(5):1614–9.
Jeru I, Le Borgne G, Cochet E, Hayrapetyan H, Duquesnoy P, Grateau G, Morali A, Sarkisian T, Amselem S. Identification and functional consequences of a recurrent NLRP12 missense mutation in periodic fever syndromes. Arthritis Rheum. 2011;63(5):1459–64.
Borghini S, Tassi S, Chiesa S, Caroli F, Carta S, Caorsi R, Fiore M, Delfino L, Lasiglie D, Ferraris C, Traggiai E, Di Duca M, Santamaria G, D’Osualdo A, Tosca M, Martini A, Ceccherini I, Rubartelli A, Gattorno M. Clinical presentation and pathogenesis of cold-induced autoinflammatory disease in a family with recurrence of an NLRP12 mutation. Arthritis Rheum. 2011;63(3):830–9.
Acknowledgments
We thank Neil McCarthy for critically reviewing the manuscript. This work was supported by Agency for Science, Technology and Research (A*STAR) of Singapore.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lia Paola Zambetti, Federica Laudisi contributed equally.
Rights and permissions
About this article
Cite this article
Zambetti, L.P., Laudisi, F., Licandro, G. et al. The rhapsody of NLRPs: master players of inflammation … and a lot more. Immunol Res 53, 78–90 (2012). https://doi.org/10.1007/s12026-012-8272-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-012-8272-z