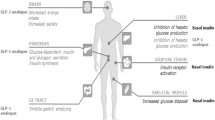
Abstract
Most patients with diabetes mellitus require multiple medications to achieve glycemic goals. Considering this and the increasing incidence of type 2 diabetes worldwide, the need for effective combination therapy is pressing. Basal insulin and glucagon-like peptide 1 (GLP-1) receptor agonists are frequently used to treat type 2 diabetes. Though both classes of medication are exclusively injectable, which may cause initial hesitation from providers, evidence for their combined use is substantial. This review summarizes the theoretical benefit, supporting evidence, and implementation of a combined basal insulin–GLP-1 receptor agonist regimen. Basal insulin added to a GLP-1 receptor agonist reduces hemoglobin A1c (HbA1c) without weight gain or significantly increased hypoglycemia. A GLP-1 receptor agonist added to basal insulin reduces HbA1c and body weight. Compared with the addition of meal-time insulin to basal insulin, a GLP-1 receptor agonist produces similar or greater reduction in HbA1c, weight loss instead of weight gain, and less hypoglycemia. Gastrointestinal adverse events are common with GLP-1 receptor agonists, especially during initiation and titration. However, combination with basal insulin is not expected to augment expected adverse events that come with using a GLP-1 receptor agonist. Basal insulin can be added to a GLP-1 receptor agonist with a slow titration to target goal fasting plasma glucose. In patients starting a GLP-1 receptor agonist, the dose of basal insulin should be decreased by 20 % in patients with an HbA1c ≤8 %. The evidence from 15 randomized prospective studies supports the combined use of a GLP-1 receptor agonist with basal insulin in a broad range of patients with uncontrolled type 2 diabetes.
Similar content being viewed by others
References
Centers for Disease Control and Prevention. In: National diabetes statistics report. http://www.cdc.gov//diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed 4 Aug 2014.
International Diabetes Federation. In: Diabetes at a glance, 2012. https://www.idf.org/sites/default/files/EUR_5E_Update_Country.pdf. Accessed 4 Aug 2014.
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40.
Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KMV, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985-2011: a modelling study. Lancet Diabetes Endocrinol. 2014. doi:10.1016/S2213-8587(14)70161-5
American Diabetes Association. Standards of medical care in diabetes 2014. Diabetes care. 2014;37(suppl 1):S14–80.
Mitka M. More patients get good diabetes control, but only a minority meet all goals. JAMA. 2013;309(13):1335–6.
Prospective UK. Diabetes Study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease: U.K. Prospective Diabetes Study Group. Diabetes. 1995;44:1249–58.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Diabetes Care. 2012;35:1364–9.
Holman RR, Farmer AJ, Davies MJ, et al. Three year efficacy of complex insulin regimens in type 2 diabetes. NEJM. 2009;361(18):1736–47.
Levemir® [package insert]. Basgvaerd, Denmark: Novo Nordisk; 2013.
Lantus® [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2013.
Gough SCL, Harris S, Woo V, et al. Insulin degludec: overview of a novel ultra-long acting basal insulin. Diabetes Obes Metab. 2013;15(4):301–9.
Petznick A. Insulin management of type 2 diabetes mellitus. Am Fam Phys. 2011;85(2):183–90.
Meneghini L, Koenen C, Weng W, et al. The usage of a simplified self-titration dosing guideline (303 algorithm) for insulin detemir in patients with type 2 diabetes-results of the randomized PREDICTIVE 303 study. Diabetes Obes Metab. 2007;9(6):902–13.
Rosenstock J, Davies M, Home PD. A randomized, 52 week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose lowering drugs in insulin-naïve people with type 2 diabetes. Diabetologia. 2008;51:408–16.
Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin. Diabetes Care. 2010;33(7):1509–15.
Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes. Ann Intern Med. 2011;154(2):103–12.
Diamant M, Nauck MA, Shaginian R, et al. Glucagon-like peptide-1 receptor agonist or bolus insulin with optimized basal insulin in diabetes. Diabetes Care. 2014. DC_140876 [pii].
Li C, Li J, Zhang Q, et al. Efficacy and safety comparison between liraglutide as add-on therapy to insulin and insulin dose-increase in Chinese subjects with poorly controlled type 2 diabetes and abdominal obesity. Cardiovasc Diabetol. 2012;11:142.
DeVries JH, Bain SC, Rodbard HW, et al. Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care. 2012;35:1446–54.
Mathieu C, Rodbard HW, Cariou B, et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON). Diabetes Obes Metab. 2014;16:636–44.
Buse JB, Vilsbøll T, Thurman J, et al. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014. DC_140785 [pii].
Rosenstock J, Fonseca VA, Gross J, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37:2317–25.
Seino Y, Min KW, Niemoeller E, Takami A. Randomized, double-blink, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14:910–7.
Riddle MC, Aronson R, Home P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin. Diabetes Care. 2013;36:2489–96.
Riddle MC, Forest T, Aronson R, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine. Diabetes Care. 2013;36:2497–503.
de Wit HM, Vervoort GMM, Jansen HJ, de Grauw WJC, de Galan BE, Tack JC. Liraglutide reverses pronounced insulin-associated weight gain, improves glycaemic control and decreases insulin dose in patients with type 2 diabetes: a 26 week, randomised clinical trial (ELEGANT). Diabetologia. 2014;57:1812–9.
Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16:827–32.
Shao N, Kuang HY, Hao M, Gao XY, Lin WJ, Zou W. Benefits of exenatide on obesity and non-alcoholic fatty liver disease with elevated liver enzymes in patients with type 2 diabetes. Diabetes Metab Res Rev. 2014;30:521–9.
Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014. S2213-8587(14)70174-3 [pii].
Byetta® [package insert]. Princeton, NJ. Brostol-Myers Squibb; 2013.
Victoza® [package insert]. Plainsboro, NJ. Novo-nordisk; 2013.
Tanzeum® [package insert]. Research Triangle Park, NC. GlaxoSmithKline; 2014.
Christensen M, Knop FK, Holst JJ, Vilsboll T. Lixisenatide, a novel GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus. IDrugs. 2009;12(8):503–13.
Creutzfeldt W. The [pre-] history of the incretin concept. Regul Pept. 2005;128:87–91.
Vilsboll T, Krarup T, Deacon C, Madsbad S, Holst J. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–13.
Shyangdan DS, Royle P, Clar C, et al. Glucagon-like peptide analogues for type 2 diabetes mellitus. Chochrane Database Syst Rev. 2011;10:CD006243.
Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes-causes, effects and coping strategies. Diabetes Obes Metab. 2007;9(6):799–812.
der Klauw Van. Wolffenbuttel. The combination of insulin and GLP-1 analogues in the treatment of type 2 diabetes. Neth J Med. 2012;70(10):436–43.
Ceriello A, Novials A, Canivell S, et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, anti-inflammatory and antioxidant action in type 2 diabetes. Diabetes Care. 2014;37:1938–43.
Eng C, Kramer C, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014. S0140-6736(14)61335-0 [pii].
Nayak UA, Govindan J, Baskar V, Kalupahana D, Singh BM. Exenatide therapy in insulin-treated type 2 diabetes and obesity. Q J Med. 2010;103:687–94.
Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39–47.
Pratley RE, Nauck MA, Barnett AH, et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2(4):289–97.
Kapitza C, Forst T, Coester HV, Poitiers F, Ruus P, Hincelin-Mery A. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab. 2013;15:642–9.
Rosenstock J, Raccah D, Koranyi L, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2013;36:2945–51.
Rosenstock J, Rodbard HW, Bain SC, et al. One-year sustained glycemic control and weight reduction in type 2 diabetes after addition ofliraglutide to metformin followed by insulin detemir according to HbA1c target. J Diabetes Complications. 2013;27:492–500.
Purnell TS, Joy S, Little E, Bridges JFP, Maruthur N. Patient preferences for noninsulin diabetes medications: a systematic review. Diabetes Care. 2014;37:2055–62.
Thong KY, Jose B, Sukumar N, et al. Safety, efficacy and tolerability of exenatide in combination with insulin in the Association of British Clinical Deabetologists nationwide exenatide audit. Diabetes Obes Metab. 2011;13:703–10.
Conflict of interest
Drs. Carris, Taylor, and Gums have no direct conflicts of interest that impacted the writing of this manuscript. No funding was received for the writing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carris, N.W., Taylor, J.R. & Gums, J.G. Combining a GLP-1 Receptor Agonist and Basal Insulin: Study Evidence and Practical Considerations. Drugs 74, 2141–2152 (2014). https://doi.org/10.1007/s40265-014-0325-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0325-2