Type 2 diabetes is associated with a high risk of CVD; consequently, the improvement of lipid profile or blood glucose control is a major challenge. Epidemiological and short-term intervention studies have highlighted that high dietary fibre intake is related to an improvement in fasting and post-prandial glycaemic control and lipid profile, associated with a lower cardiovascular risk(Reference Salmeron, Ascherio and Rimm1–Reference Liu, Buring and Sesso3). In this context, soluble dietary fibre supplementation has been considered as a therapeutic tool to improve these parameters.
β-Glucan, a non-starch viscous polysaccharide derived from oat, has been shown to reduce total cholesterol (TC) and LDLc levels, and post-prandial glucose and insulin response(Reference Braaten, Wood and Scott4–Reference Tappy, Gugolz and Wursch7). Several mechanisms are proposed as to explain the cholesterol-lowering effect of β-glucan: reduced cholesterol absorption due to an increased meal bolus viscosity, reduced cholesterol synthesis due to an increased conversion of cholesterol to faecal bile acids and/or an inhibition of hepatic fatty acid synthesis by products of fermentation(Reference Lia, Hallmans and Sandberg8–Reference Naumann, van Rees and Onning10). Concerning glucose metabolism, the beneficial metabolic effects of oat β-glucan are tightly linked to the β-glucan-induced increased viscosity of the meal bolus, which delays and/or reduces carbohydrates absorption(Reference Battilana, Ornstein and Minehira11, Reference Wood, Braaten and Scott12).
However, the increase in intestinal viscosity is dependant of β-glucan molecular weight and solubility, food processing, β-glucan concentration and associated food matrix. β-Glucan enrichment of food products has been largely studied, because it permits a higher intake per serving with a minimum decrease in palatability(Reference Jenkins, Kendall and Vuksan13). So, to reach the 3 g/d intake recommended by Food and Drug Administration, consumers must be offered a range of palatable and sufficiently enriched products. Up to now, few studies have specifically focused on the effect of β-glucan as an adjunct therapeutic aid for metabolic control in type 2 diabetic subjects in the mid-term range(Reference Reyna, Cano and Bermudez14). In parallel to the numerous epidemiological and short-term intervention studies concerning dietary fibre and diabetes, evidence-based studies investigating long-term efficiency of β-glucan enrichment in type 2 diabetic subjects are missing.
The present work aims at analysing the effects of the enrichment of a normal diet with reasonable amount of β-glucan (3·5 g/d) on glucose control (HbA1c level) and on lipid profile (TC, HDL cholesterol, LDLc and apo B) for 2 months in a representative group of free-living type 2 diabetic subjects, with different treatments (anti-diabetic, lipid-lowering, diet) and characteristics (time from diagnosis, sex and age). Using a palatable ready-to-eat frozen soup seemed to be a good alternative to maintain both physiological effects and good sensory properties and to implement an everyday dietary intervention over a long-term period.
Subjects and methods
Study design
The present study was a parallel, placebo-controlled, blinded randomised trial. For a run-in period of 3 weeks, the subjects consumed one control soup a day (without β-glucan). For the following 8-week intervention period, one group continued to consume the control soup (control group), while the other group consumed the soup containing 3·5 g β-glucan (β-glucan group).
At the beginning of the run-in period (week (W) 0), of the dietary intervention (W3) and at the end (W11), body weight was measured and fasting blood samples were collected. The subjects completed a 5-d dietary questionnaire on W0 and W11.
Study population
The type 2 diabetes patients were recruited in Lund and Lyon, and a medical examination and screening visit were performed prior involvement, received written and oral information about the protocol and signed an informed consent form. The study was approved by the Scientific Ethics Committees in Lyon (CCPPRB Lyon A) and Lund and accorded with both the French ‘Huriet-Serusclat’ law and the Second Declaration of Helsinki. The inclusion criteria were men or women aged 30–75 years old, BMI 20–35 kg/m2, stable body weight, with an HbA1c < 11 %. The exclusion criteria were pregnancy, breastfeeding, severe renal complications, secondary dyslipidaemia, TAG >4 mmol/l, anaemia, treatment by orlistat, pancreatic disease and malignancy>1 year ago.
Experimental products and dietary regimen
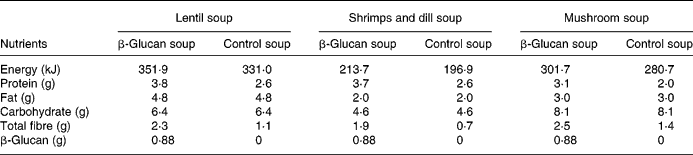
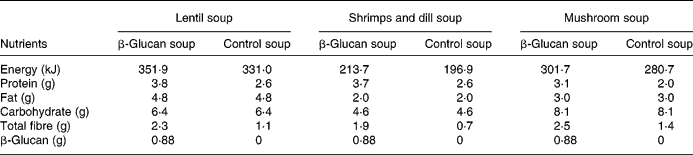
The experimental products were frozen ready-to-eat soups (Findus R&D, Bjuv, Sweden) with three different flavours: lentil and ham; shrimps and dill; mushroom (composition in Table 1). A soluble oat concentrate of β-glucan (3·5 g) was added to the enriched soups (Ceba Foods, Lund, Sweden). Attempts were made to increase further the concentration of β-glucan, but this negatively affected the palatability. The soups composition has been adjusted to obtain levels with respect to carbohydrates and lipids, by adding rapeseed oil to control soup and maltodextrin to β-glucan soup. β-Glucan in the soup has a molecular weight of 80 kDa, and preparation, freezing and storage did not alter the molecular weight (analysis by VTT Biotechnology, Espoo, Finland).
The subjects were instructed to consume daily one soup as the main component of a lunch or dinner meal and if desired add drink and/or other food products. Subjects and investigators were blinded for the type of soup ingested.
As a measure of compliance, packages that were left over must be returned and were counted.
Subjects were also asked not to change their usual food habit, physical activity and to record any signs of illness, medication used or deviation to the protocol.
Food habits were assessed once before the run-in period and once at the end of the intervention by a 5-d food intake questionnaire and analysed by a trained registered dietitian (in Lyon, using a SUpplémentation en VItamines et Minéraux AntioXydants© dietary photographic support and in Lund, a food database from the Swedish National Food Administration (PC-Kost 1_99, SLV, Uppsala, Sweden).
Testing and analytical procedures
Subjects came to the research centre at the start of the study (W0), at the end of the run-in period (W3), at the middle of the study (W7) and at the end of the study (W11). Body weight was measured using a calibrated scale. Blood samples were drawn following an overnight fast, collected in tubes maintained at 4°C and immediately centrifuged at 4500 rpm for 10 min at 4°C. Plasma was then stored at − 20°C until assay.
Fasting glucose concentrations, HDL cholesterol, TC and TAG were analysed with respective enzymatic kits from Roche Diagnostics using an autoanalyzer (Roche Diagnostics Hitachi 917; Hitachi, Tokyo). LDLc was calculated by the Friedewald formula(Reference Friedewald, Levy and Fredrickson15). Plasma concentrations of apo B were determined by immunonephelometry using an Immage Beckman instrument (Beckman Instruments, Fullerton, CA, USA). HbA1c levels were determined by HPLC (Biorad, Marnes la Coquette, France).
Statistical analysis
Results are given as means and standard deviations except TC, LDLc, HbA1c and fasting glucose when plotting in graphs (standard error of the mean). Changes in parameters according to group assignment were calculated as the change and the percentage of change between values at the end of the run-in period (W3) and at the end of the intervention period (W11).
Equality of variance and distribution normality have been checked prior further analysis. Differences between groups at baseline and change and percentage of change between W3 and W11 were assessed using an unpaired t test. In case of non-normal distribution, a non-parametric Mann and Whitney test was done. The present study including thirty subjects in each group has the power to detect a significant decrease in Hba1c lower or equal to 0·5 % with a standard deviation = 0·7 % (β-risk of 20 % and α-risk of 5 %; P Moulin, unpublished clinical). Statistical significance was inferred at P < 0·05. All statistical analyses were performed using Statview version 5.0 (SAS Institute, Cary, NC, USA) software.
Results
Subjects
Sixty-seven diabetic volunteers were screened in Lyon and Lund and were randomly assigned to either the control group or the β-glucan group. There was no centre effect for any studied parameter. Only fifty-three subjects (twenty-four subjects in the control group and twenty-nine subjects in the β-glucan group) were included in the analysis due to lipid profile anomaly (two subjects: TAG >4 mmol/l at W0) or withdrawal (twelve subjects). The population was composed of twenty-one women and thirty-two men with mild obesity. About 50 % of the subjects were under lipid-lowering treatment (twelve in the control group and fifteen in the β-glucan group). As for anti-diabetic treatment, subjects were either under insulin or medication treatment (twelve in the control group and nine in the β-glucan group), either under diet and/or medication treatment (twelve in the control group and twenty in the β-glucan group). Importantly, the treatments were kept constant throughout the study. The subjects' characteristics are given in Table 2. They were normocholesterolaemic with a mild hypertriglyceridaemia and a fair blood glucose control. Due to randomisation, both groups were comparable, except for a higher concentration of plasma TAG in the β-glucan group.
Table 2 Baseline characteristics of the study subjects according to group
(Mean values and standard deviations)
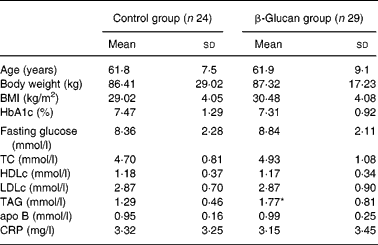
TC, total cholesterol; HDLc, HDL cholesterol; LDLc, LDL cholesterol; CRP, C-reactive protein.
* Mean values were significantly different between β-glucan and control groups (Mann–Whitney test; P = 0·02).
Side effects and compliance
All the participants followed the experimental protocol without difficulty. The compliance was good (based on soup packages count). The soup portion was mostly ingested at dinner in the two groups.
There were no significant differences between the groups in the safety parameters: number of results leucocytes, erythrocytes and platelets; or serum concentration of CRP, alanine aminotransferase, aspartate aminotransferase, γ-glutamyltransferase and creatinine (data not shown). There was no record of side effects as headache, stomach complaints, nausea, flatulence, diarrhoea, bloated feeling, eruption/rashes, fatigue and dizziness. The twelve withdrawals, with a similar distribution in both groups, were not due to diet intolerance or side effects, but to personal reasons.
Run-in period
During the 3-week run-in period, none of the measured metabolic parameters was modified in the two groups (data not shown).
Anthropometric parameters and diet
The mean baseline BMI in both groups (Table 2) remained constant throughout the study and did not significantly differ between groups: +0·18 (sd 1·33) kg/m2 in the β-glucan group v. +0·36 (sd 1·37) kg/m2 in the control group during intervention period (P = 0·63; data not shown).
The mean daily dietary intakes before the run-in period (W0) were measured: 6·9 (sd 1·6) MJ of total energy; 34·3 (sd 6·2) % of total energy as fat; 19·0 (sd 2·4) % of total energy as protein; 44·1 (sd 5·4) % of total energy as carbohydrates; 19·7 (sd 5·3) g of dietary fibres for the β-glucan group v. 8·2 (sd 3·0) MJ of total energy; 35·2 (sd 5·5) % of total energy as fat; 17·9 (sd 2·7) % of total energy as protein; 43·7 (sd 7·4) % of total energy as carbohydrates; 22·3 (sd 12·0) g of dietary fibres for the control group. The registered energy and nutrient intake remained stable throughout the study, and there were no significant differences in either group at the end of the intervention period (W11). However, a trend toward a mild decrease (NS) in fibre consumption during the intervention period was noticed in the two groups.
Serum lipids
The blood lipid profile was unchanged during the β-glucan dietary intervention (Table 3). TC and LDLc decreased in the β-glucan group, but this decrease was NS compared with the control group (Fig. 1(a) and (b)). The apo B concentration remained also stable in both groups. HDL cholesterol increased significantly between W0 and W11: +5·37 (sd 10·47) % (P = 0·01) in the β-glucan group. No significant difference was found in HDL cholesterol/LDLc ratio between W3 and W11 in the two groups (data not shown). The serum TAG decreased by − 3·75 (sd 22·2) % in the β-glucan group and increased by +11·24 (sd 32·40) % in the control group. This difference between the two groups was significant, even after adjustment for TAG concentration at W0 or W3 as covariant (P = 0·03). Additionally, the lipid profile's changes were not altered by the presence or absence of a lipid-lowering drug treatment.
Table 3 Change in total cholesterol (TC), LDL- (LDLc), HDL-cholesterol (HDLc), TAG, apo B, HbA1c and fasting glucose*
(Mean values and standard deviations)

Change (%), percentage change.
* When the run-in period values were used as covariates, the significance level (P) and interpretation of the results did not change.
† Mean values were significantly different between β-glucan and control groups (P < 0·05; Mann–Whitney test or unpaired t test).

Fig. 1 Mean concentrations with their standard errors of mean during the run-in period (week (W0–W3) and the intervention period (W3–W11) for β-glucan group (▲, dotted line; n 29) and for control group (■, plain line; n 24). (a) Total cholesterol (TC; mmol/l); (b) LDL cholesterol (LDLc; mmol/l); (c) HbA1c (%); (d) fasting glycaemia (mmol/l). No significant difference was observed between the two groups.
Glycaemic control
The primary glycaemic end point, HbA1c, was not reduced during intervention period in the β-glucan group (Table 3; Fig. 1(c)). The W11–W3 HbA1C change was not different between the two groups. The mild increase in fasting glucose over the duration of the study was NS and similar in both groups (Table 3; Fig. 1(d)).
Discussion
The present study shows that the long-term consumption of a β-glucan-enriched soup once daily as the main part of the meal had no detectable effect on the metabolic profile in free-living type 2 diabetic subjects. The incorporation of 3·5 g of β-glucan in soups, ingested for 8 weeks, did not alter plasma LDLc and HbA1c. The targeted population consisted of a representative group of free-living type 2 diabetes patients. Indeed, the fifty-three subjects (44–75 years old) had a time from diabetes diagnosis ranging between 1 and 25 years. They were under different anti-diabetic treatment (diet, sulphonylurea, metformin, repaglinide and α-glycosidase inhibitor) and/or lipid-lowering treatment (fibrates and statins).
The present study was unable to provide evidence of a significant improvement in lipid profile. It has been shown that 3 g soluble fibre could decrease TC and LDLc(Reference Brown, Rosner and Willett6). According to the meta-analysis of Brown et al. (Reference Brown, Rosner and Willett6), a reduction of − 0·04 mmol/l per g of fibre for LDLc would have been expected, so for 3·5 g of oat β-glucan − 0·12 mmol/l. In contrast, β-glucan-enriched beverages (fruit drink and oat milk) did improve the lipid profile in the expected magnitude predicted from this meta-analysis(Reference Naumann, van Rees and Onning10, Reference Onning, Wallmark and Persson16). However, several other studies failed to demonstrate an effect of β-glucan incorporation into a solid matrix on the lipid profile in hypercholesterolaemic subjects(Reference Torronen, Kansanen and Uusitupa17–Reference Biorklund, Holm and Onning19). Thus, 5·9 g β-glucan incorporated into cookies and bread during 4 weeks did not significantly change TC and LDLc(Reference Kerckhoffs, Hornstra and Mensink18). The meta-analysis of Ripsin et al. (Reference Ripsin, Keenan and Jacobs20) suggested that a larger reduction of TC and LDLc were seen in subjects with higher cholesterol levels, particularly when a dose of 3 g or more of oat fibre was used. However, Biorklund et al. (Reference Biorklund, Holm and Onning19) recently has not shown any effect with 4 g β-glucan incorporated into a soup in forty-three hyperlipidaemic subjects. Considering the low LDLc encountered in type 2 diabetes subjects, well controlled for lipid profile, the decrease in LDLc was expected to be weaker. Moreover, half of our subjects were treated with a lipid-lowering treatment, which could have blunted the fibre effect, but results were not modified in the subgroup without lipid-lowering treatment (data not shown). Surprisingly, the present results showed for the first time a significant diminution of TAG concentration in the β-glucan group. It is likely that it is the consequence of the regression toward the mean phenomenon, since the β-glucan group had a higher level of plasma TAG at baseline.
Few studies have assessed the efficacy of fibres on the lipid profile in type 2 diabetes. In a small group of type 2 diabetic subjects, fed a high amount of soluble fibre (7 g guar gum three times a day for 3 months), a significant reduction in LDLc (from 5·19 mmol/l (sem 0·34) to 4·30 (sem 0·40) mmol/l) was found(Reference Aro, Uusitupa and Voutilainen21). Also, another crossover study in eight type 2 diabetic men has shown a decrease in LDLc from 3·36 (sem 0·12) to 2·59 (sem 0·12) mmol/l with 9 g oat bran fibre incorporated into bread, buns or muffins during 12 weeks(Reference Pick, Hawrysh and Gee22). The higher beneficial effect was observed with ingestion of 8–13 g konjac-mannan-enriched biscuits associated to a low-fat diet during 3 weeks (LDLc from 3·89 (sem 0·25) to 3·04 (sem 0·24) mmol/l)(Reference Vuksan, Jenkins and Spadafora23).
Concerning glucose control, the anti-diabetic effects of β-glucans have been suspected in regards to their actions on energy and glucose metabolism and to their lowering effect on post-prandial glycaemic and insulinaemic responses in healthy and fewer in type 2 diabetic subjects(Reference Tappy, Gugolz and Wursch7, Reference Battilana, Ornstein and Minehira11, Reference Reyna, Cano and Bermudez14, Reference Braaten, Scott and Wood24–Reference Nazare, Normand and Oste Triantafyllou26). Up to now, only one study investigated the effect of a diet enriched in β-glucan on HbA1c in type 2 diabetes, in addition to the recommended American Diabetes Association diet(Reference Reyna, Cano and Bermudez14). Eight diabetic patients (mean HbA1c of 8·5 %) followed a 4-week low-energy diet, enriched with 8 % of β-glucan. Fasting glycaemia did not change, but a weak decrease in HbA1c was observed in the β-glucan group. But β-glucan enrichment was associated to the replacement of sugars by sweeteners. In our diabetic group, the lower baseline HbA1c (7·38 (sd 1·10) %) may explain the lack of significant effect of soups used as the exclusive intervention. Another fibre, konjac-mannan, significantly decreased fructosamine in diabetic subjects(Reference Vuksan, Jenkins and Spadafora23). In the present study, fructosamine analysis was performed between W0 and W11 (data not shown), and no change was observed.
Concerning fasting glycaemia, we did not found any significant effect of β-glucan. There was a trend for a decrease in fasting glycaemia in subgroup without insulin injection (NS). Aro et al. (Reference Aro, Uusitupa and Voutilainen21) showed a significant decrease in fasting and post-prandial hyperglycaemia in type 2 diabetic subjects following a 3-month supplementation (three times 7 g guar gum daily).
Considering study design using dietary supplementation in free-living subjects, discrepancies between results can thus partly be explained by differences in food matrix, amount of β-glucan ingested, ingestion frequency, HbA1c level and lipid status at baseline, intervention duration and population size. It is well known that the fibre type, the interactions with other constituents in the composite food matrix or the mode of administration are likely to influence structural features, concentration and solubility of β-glucans and consequently modulate their physiological action. The efficiency of β-glucan to alter metabolic parameters such as cholesterol level is higher when β-glucan is part of a liquid food matrix compared to a solid one(Reference Naumann, van Rees and Onning10, Reference Onning, Wallmark and Persson16, Reference Kerckhoffs, Hornstra and Mensink18, Reference Anderson, Davidson and Blonde27). Moreover, one study has been done in order to verify the willingness of consumers to use beverages and ready-to-eat frozen soups containing oat β-glucan(Reference Lyly, Honkapää and Poutanen28). Although β-glucan addition altered the sensory characteristics of both the beverages and soups, willingness to use soups was not remarkably lowered. Whereas soups seemed to be a good vector of administration, well tolerated, palatable and convenient, the acceptable amount of β-glucan (3·5 g/serving) may not be sufficient to induce a physiological effect on lipid or glucose profile. More than 3·5 g per serving, we encountered high decrease in palatability, which could have compromised the good compliance to the study and a possible future extension in the longer-term. Finally, the frequency of ingestion may have negatively influenced the present results since the β-glucan soup was ingested only once a day compared to other studies(Reference Onning, Wallmark and Persson16, Reference Vuksan, Jenkins and Spadafora23). Besides, β-glucan soups were ingested as main part of a meal and may have replaced other fibre-rich food as vegetables or fruits in the dietary pattern.
In conclusion, this 2-month intervention trial in a large group of type 2 diabetic subjects shows that a single daily ingestion of a soup enriched with 3·5 g β-glucan (according to official recommendations) does not improve the lipid profile and HbA1c level in free-living conditions. Long-term benefits from β-glucan-enriched foods in the diabetic population might occur with higher doses and/or increased frequency of intake, but such consideration raises the question of the supplementation acceptability.
Acknowledgements
The present work was a part of the European project QLKI-2000-00 535 ‘Design of food with improved functionality and superior health effects using cereal β-glucans’ supported by the European Commission. It does not necessarily reflect its views and in no way anticipates the Commission's future policy in this area. Additional support was obtained from the Påhlsson Foundation and the Swedish Governmental Agency for Innovation Systems (to G. O.). J. H. is employee of Findus.
We thank all the staff of the Centre de Recherche en Nutrition Humaine Rhône-Alpes and of the Unit for diabetic studies (Lund University) for their excellent technical assistance in subject recruitment, sample collection and analysis, and dietary surveys, Dr T. Suortti, Dr M. Salmenkallio-Marttila from VTT Biotechnology for analysis of the study products, Findus R&D for the frozen soups production, Picard Surgelés (France) for the frozen soups supply and all the subjects for taking part in this trial.
C. C.-A. and J.-A. N. were responsible for data collection and analysis, statistical analysis, writing and evaluation of the paper. M. B. and E. L. C. were responsible for study coordination, data collection and analysis. A. S. was responsible for biochemical assays. M. S. was responsible for dietary surveys and analysis. J. H. worked at Findus and provided expertise and knowledge in test products. M. L.-O. was responsible for subject involvement and coordination of subject management. M. L., G. O. and P. M. were responsible for study coordination, study design and supervised the writing of the paper.