Abstract
Objective:
Fibroblast growth factor 21 (FGF21) reduces plasma glucose and triglycerides, and increases free fatty acid oxidation in animal models of diabetes. The aim of the present study was to assess the relationships of serum FGF21 with glucose oxidation (GOx) and lipid oxidation (LOx) in the baseline and insulin-stimulated conditions in lean and obese subjects.
Design:
Cross-sectional study.
Subjects:
Eighty-four subjects with normal glucose tolerance, 42 lean (body mass index (BMI) <25 kg m−2) and 42 overweight or obese (BMI between 25 and 40 kg m−2).
Measurements:
Euglycemic hyperinsulinemic clamp and indirect calorimetry in the baseline state and during last 30 min of the clamp. The change in respiratory quotient (ΔRQ) in response to insulin was used as a measure of metabolic flexibility. Serum FGF21 was determined in the baseline state and after the clamp.
Results:
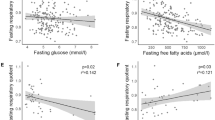
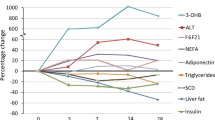
Obese subjects had higher LOx in the baseline and insulin-stimulated conditions, lower insulin-stimulated GOx and ΔRQ (all P<0.05). Fasting serum FGF21 did not differ between the groups. Insulin infusion resulted in an increase in serum FGF21 in the obese (P=0.0001), but not in the lean group (P=0.76). Postclamp serum FGF21 was higher in the obese subjects (P=0.0007). In this group, postclamp FGF21 was related to LOx during the clamp (r=0.32, P=0.044), change in GOx and LOx in response to insulin (r=−0.44, P=0.005; r=0.47, P=0.002; respectively) and ΔRQ (r=−0.50, P=0.001).
Conclusions:
An increase in serum FGF21 in response to insulin in obese subjects might represent inappropriate response, possibly associated with metabolic inflexibility in obesity and insulin resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ et al. FGF-21 as a novel metabolic regulator. J Clin Invest 2005; 115: 1627–1635.
Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E . Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007; 5: 426–437.
Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R et al. FGF21 induces PGC1α and regulates carbohydrate metabolism during the adaptive starvation response. PNAS 2009; 106: 10853–10858.
Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab 2007; 5: 415–425.
Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009; 58: 25–259.
Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008; 57: 1246–1253.
Chavez AO, Molina-Carrion M, Abdul-Ghani M, Folli F, DeFronzo RA, Tripathy D . Circulating fibroblast growth factor 21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 2009; 32: 1542–1546.
Chen WW, Li L, Yang GY, Li K, Qi XY, Zhu W et al. Circulating FGF-21 levels in normal subjects and in newly diagnosed patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 2008; 116: 65–68.
Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010; 139: 456–463.
Li H, Bao Y, Xu A, Pan X, Lu J, Wu H et al. Serum fibroblast growth factor 21 is associated with adverse lipid profiles and γ-glutamyltransferase but not insulin sensitivity in Chinese subjects. J Clin Endocrinol Metab 2009; 94: 2151–2156.
Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 2010; 59: 2781–2789.
Hale C, Chen MM, Stanislaus S, Chinookoswong N, Hager T, Wang M et al. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology 2012; 153: 69–80.
Mai K, Andres J, Biedasek K, Weicht J, Bobbert T, Sabath M et al. Free fatty acids link metabolism and regulation of insulin-sensitizing fibroblast growth factor 21. Diabetes 2009; 58: 1532–1538.
Hojman P, Pedersen M, Nielsen AR, Krogh-Madsen R, Yfanti C, Akerstrom T et al. Fibroblastgrowth factor 21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes 2009; 58: 2797–2801.
Mraz M, Bartlova M, Lacinova Z, Michalsky D, Kasalicky M, Haluzikova D et al. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol 2009; 71: 369–375.
Vienberg SG, Brons C, Nilsson E, Astrup A, Vaag A, Andersen B . Impact of short-term high-fat feeding and insulin-stimulated FGF21 in subjects with low birth weight and controls. Eur J Endocrinol 2012; 167: 49–57.
Mai K, Bobbert T, Groth C, Assmann A, Meinus S, Kraatz J et al. Physiological modulation of circulating FGF21: relevance of free fatty acids and insulin. Am J Physiol 2010; 299: E126–E130.
Yang M, Dong J, Liu H, Li L, Yang G . Effects of short-term continuous subcutaneous insulin infusion on fasting plasma fibroblast growth factor-21 levels in patients with newly diagnosed type 2 diabetes mellitus. PLoS ONE 2011; 6: e26359.
Kelley DE . Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest 2005; 115: 1699–1702.
Karczewska-Kupczewska M, Kowalska I, Nikołajuk A, Adamska A, Zielińska M, Kamińska N et al. Circulating brain-derived neurotrophic factor concentration is downregulated by intralipid/heparin infusion or high-fat meal in young healthy male subjects. Diabetes Care 2012; 35: 358–362.
DeFronzo RA, Tobin JD, Andres R . Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214–E223.
Karczewska-Kupczewska M, Strączkowski M, Adamska A, Nikołajuk A, Otziomek E, Górska M et al. Insulin sensitivity, metabolic flexibility, and serum adiponectin concentration in women with anorexia nervosa. Metabolism 2010; 59: 473–477.
Acknowledgements
This work was supported by Grant UDA-POIG.01.03.01-00-128/08, from the Program Innovative Economy 2007–2013, part-financed by the European Union within the European Regional Development Fund, and grant 3-50689 from Medical University of Białystok, Poland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Strączkowski, M., Karczewska-Kupczewska, M., Adamska, A. et al. Serum fibroblast growth factor 21 in human obesity: regulation by insulin infusion and relationship with glucose and lipid oxidation. Int J Obes 37, 1386–1390 (2013). https://doi.org/10.1038/ijo.2013.10
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2013.10
Keywords
This article is cited by
-
Deficiency of fibroblast growth factor 21 aggravates obesity-induced atrophic responses in skeletal muscle
Journal of Inflammation (2019)
-
Fasting and glucose induced thermogenesis in response to three ambient temperatures: a randomized crossover trial in the metabolic syndrome
European Journal of Clinical Nutrition (2018)
-
Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients
International Journal of Obesity (2015)
-
Obesity and carotid artery remodeling
Nutrition & Diabetes (2015)
-
The role of the Nrf2/Keap1 pathway in obesity and metabolic syndrome
Reviews in Endocrine and Metabolic Disorders (2015)