Abstract
Dyslipidemia is one of the most common adverse effects in schizophrenia patients treated with antipsychotics. However, there are no established effective treatments. In this study, data were pooled from two randomized, placebo-controlled trials, which were originally designed to examine the efficacy of metformin in treating antipsychotic-induced weight gain and other metabolic abnormalities. In total, 201 schizophrenia patients with dyslipidemia after being treated with an antipsychotic were assigned to take 1000 mg day–1 metformin (n=103) or placebo (n=98) for 24 weeks, with evaluation at baseline, week 12 and week 24. The primary outcome was the low-density lipoprotein cholesterol (LDL-C) levels. After metformin treatment, the mean difference in the LDL-C value between metformin treatment and placebo was from 0.16 mmol l–1 at baseline to –0.86 mmol l–1 at the end of week 24, decreased by 1.02 mmol l–1 (P<0.0001); and 25.3% of patients in the metformin group had LDL-C ≥3.37 mmol l–1, which is significantly <64.8% in the placebo group (P<0.001) at week 24. Compared with the placebo, metformin treatment also have a significant effect on reducing weight, body mass index, insulin, insulin resistance index, total cholesterol and triglyceride, and increasing high-density lipoprotein cholesterol. The treatment effects on weight and insulin resistance appeared at week 12 and further improved at week 24, but the effects on improving dyslipidemia only significantly occurred at the end of week 24. We found that metformin treatment was effective in improving antipsychotic-induced dyslipidemia and insulin resistance, and the effects improving antipsychotic-induced insulin resistance appeared earlier than the reducing dyslipidemia.
Similar content being viewed by others
Introduction
A large body of literature show that antipsychotics, particularly atypical antipsychotics cause serious adverse metabolic effects, which include dyslipidemia, weight gain, insulin resistance and glucose intolerance.1, 2 According to a recent study in an Asia population, 66% of patients with chronic schizophrenia had dyslipidemia after treatment with an antipsychotic,3 although there is no difference between typical and atypical antipsychotics. Patients treated with clozapine and olanzapine are more likely to have weight gain,4 and elevated level of insulin and insulin resistance.5 Dyslipidemia is one of major factors leading to cardiovascular diseases, such as myocardial infarction and stroke.6 Patients with schizophrenia are twice as likely dying from cardiovascular disease as the general population, and tend to have an average of 9–12 years of life loss.7, 8 The development of cardiovascular illness and early death in patients with schizophrenia are to a certain degree attributed to dyslipidemia.
Dyslipidemia is an imbalance of individual’s lipid components, comprising elevated level of total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglycerides, or reduced level of high-density lipoprotein cholesterol (HDL-C). It is generally believed that elevated LDL-C is directly associated with cardiovascular events, and is the primary target of lipid-lowering therapy.9 In China, Europe and the United States, guidelines for lower LDL-C levels are recommended; and aggressively lowering the level of LDL-C becomes particularly important.10, 11, 12 Dyslipidemia has become a major concern in the treatment of psychosis. However, there is a relative lack of interventions to control antipsychotic-induced dyslipidemia.
Metformin, a biguanide, is a well-known for its use of treating type 2 diabetes mellitus.13 In our previous study, metformin can improve antipsychotic-induced weight gain and insulin resistance.14, 15, 16 Insulin resistance is recognized as an important metabolic defect linking the components of metabolic syndrome that includes atherogenic dyslipidemia,17 and induces systemic cardiovascular risk such as hypertension18 and hyperglycemia.19 The link between insulin resistance and dysregulation of lipoprotein metabolism is well established.20, 21, 22
An animal study has found that metformin effectively ameliorate the development of risperidone-induced insulin resistance and dyslipidemia (that is, altered serum triglyceride and total cholesterol levels), which is mediated via improvement in insulin resistance.23 In diabetic patients, metformin treatment appears to have some beneficial effects on circulating lipid levels by decreasing the concentrations of plasma triglycerides and LDL-C, increasing the level of HDL-C and ratio of HDL-C to LDL-C.24 However, these effects depend on the improvement of insulin resistance. Therefore, it is reasonable to assume that metformin may attenuate antipsychotic-induced dyslipidemia through improving insulin resistance.
However, it remains unclear if metformin is effective in improving antipsychotic-induced dyslipidemia in patients who have weight gain and/or have developed insulin resistance after antipsychotic treatment. Whether or not the effect of metformin treatment on antipsychotic-induced dyslipidemia is attributed to the improvement of insulin resistance. Therefore, we designed this study to examine the effect of metformin treatment on antipsychotic-induced dyslipidemia and to determine the role of insulin resistance in metformin treatment-related changes in lipid profile in patients with schizophrenia.
Materials and methods
Study design and data
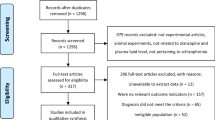
The study was designed using two independent double-blind, randomized, placebo-controlled 6-month clinical trials of metformin in antipsychotic-induced dyslipidemia, weight gain and other metabolic changes. Although the first trial (STUDY 1, NCT01778244; recruitment, 2008–2012) was to examine the efficacy of metformin for treatment of antipsychotic-induced dyslipidemia; the second trial (STUDY 2, NCT01206153) was initially designed to investigate the efficacy of metformin in the treatment of antipsychotic-induced amenorrhea and weight gain. The data from the STUDY 1 have not been published elsewhere, although the primary outcomes of the STUDY 2 have been published.14
Two studies were identical in terms of measurements on lipids, weight gain and other serum chemicals. In STUDY 1, patients had to have an antipsychotic-induced dyslipidemia to meet the eligibility for the study; whereas in STUDY 2, all patients were women with antipsychotic-induced amenorrhea. Both studies were conducted in the Mental Health Institute of the Second Xiangya Hospital at the Central South University, China and two data sets were combined after that initial examination found little difference in related demographics and metabolic outcome measurements at baseline. Total 162 patients from STUDY 1 and 39 patients (all females) from STUDY 2, after excluding 45 patients who did not have dyslipidemia at the beginning of STUDY 2, were included for this analysis.
Participants
Patients aged 18–40 years with a first psychotic episode of schizophrenia diagnosed in accordance with criteria set out in the Diagnostic and Statistical Manual of Mental Disorders- Fourth Edition (DSM-IV) were eligible for our study,25 and the Structured Clinical Interview of DSM-IV Axis I Disorders (SCID-1), Clinical Version, was used during the screening phase.26 To be qualified for the STUDY 1, patients had to meet: (1) have experienced dyslipidemia as defined below within the first year of treatment with one of four antipsychotics—clozapine, olanzapine, risperidone or sulpiride; (2) had been either discharged from inpatient units or first visit in the outpatient clinic in the 12 months before enrollment, and their lipid level, weight and antipsychotic treatment were documented; (3) had taken single antipsychotic medication, with no more than a 25% change in dosage, over the past 3 months. Dyslipidemia was defined on the basis of the Chinese guidelines for dyslipidemia in clinical practice using total cholesterol, triglyceride, HDL-C and LDL-C to guide treatment. It was defined as normal (no dyslipidemia) if total cholesterol <5.18 mmol l–1 (200 mg dl–1), triglyceride <1.70 mmol l–1 (150 mg dl–1), HDL-C ⩾1.04 mmol l–1 (40 mg dl–1) and LDL-C <3.37 mmol l–1 (130 mg dl–1);10 otherwise as dyslipidemia if patients had exceeded the threshold in either of four lipid measures. All participants had to be under the care of a parent or another adult caregiver who monitored and recorded the intake of medication daily during the trial to assure the adherence to medication. Patients were excluded from the study if there was evidence of liver or renal dysfunction, cardiovascular disease or diabetes mellitus; or if they were pregnant or lactating; or had any psychiatric diagnosis other than schizophrenia. For the STUDY 2, the inclusion and exclusion criteria have been previously described;14 and it followed the similar protocol as in the STUDY 1. All participants were followed for 24 weeks after starting metformin treatment.
Pharmacological intervention
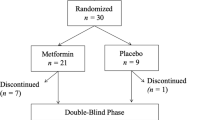
Participants were randomly assigned to receive metformin (1000 mg day–1) or placebo for up to 6 months. The dosage of 1000 mg day–1 metformin was based on safety and efficacy for Chinese diabetic patients with dyslipidemia.27 For the first 3 days, participants took 250 mg of metformin or placebo twice a day, one before lunch and one before dinner. From day 4 onward, they took 500 mg of metformin or placebo twice a day. Any antipsychotics that patients were taking before enrollment in the study remained at the same dosage throughout the course of the study. Only trihexyphenidyl for extrapyramidal symptoms and lorazepam for insomnia or agitation were considered concomitant medications and were allowed as needed during the study period. Patients' adherence to the study medication for each visit was defined as having taken >80% of the study drug dose prescribed for that period. If a participant was not adherent, both patient and caregiver were counseled on the importance of taking the prescribed medications of study.
Primary and secondary outcome measurements
The primary outcome was the level of LDL-C or the percentage of patients who had LDL-C ≥3.37 mmol l–1 (130 mg dl–1). The secondary outcomes were fasting triglyceride, total cholesterol, HDL-C, insulin and glucose levels, insulin resistance index, body weight, body mass index and Positive and Negative Syndrome Scale (PANSS) score, as well as incidence of adverse events. Body mass index was calculated as weight in kilograms divided by height in meters squared. Insulin resistance index was calculated based on the homeostasis model assessment28 as: fasting insulin (mIU l–1) × fasting glucose (mmol l–1)/22.5.
The baseline data included related demographics, a comprehensive medical history, clinical evaluations of psychiatric symptoms, a physical examination with measurements of weight and height, and related laboratory tests. The PANSS29 and the Treatment Emergent Symptom Scale30 were used for monitoring psychiatric symptoms and adverse effects. Research nurses who performed all assessments were blind to the type of treatments. The baseline laboratory tests included level of fasting LDL-C, triglyceride, cholesterol, HDL-C, glucose and insulin, lactic acid in serum, liver and renal function tests, blood counts and electrocardiogram. Fasting blood samples were drawn after confirmation with patients or their caregivers.
Follow-up visits were made at weeks 12 and 24 after starting treatment. At each follow-up visit, all baseline evaluations including physical examination, laboratory tests, weight and height measurements, and Treatment Emergent Symptom Scale were repeated. The PANSS was administered again at week 24. Lipid levels, glucose and lactic acid levels, liver and renal function tests were determined by enzymatic procedures of the Boehringer Mannheim/Hitachi 714 automated chemistry analyzer (Hitachi, Tokyo, Japan). Serum insulin level was measured with a solid-phase radioimmunoassay. Weight and height measurements were made after participants removed their shoes and upper garments, and donned an examination gown.
Statistical analysis
Single variate descriptive analysis was performed to examine the difference in demographics and metabolic measures at baseline between two trials and two treatment groups in the combined data. Necessary statistical tests were performed, in which χ2 test was for categorical variables; and t-test and general linear model while adjusting for related covariates were used to test for continuous variables at the baseline. Analysis of within-group time effect and modeling the treatment effect by time while controlling for age, sex and duration of illness was performed using general linear mixed model, which is an effective way to analyze quantitative outcomes before and after treatment in clinical trial;31, 32 and post-hoc least square estimates of mean were obtained based on modeling results. The difference was considered statistically significant if a two-tailed P-value was <0.05. Analysis was performed using SAS 9.13 (SAS Inc., Carry, NC, USA).
Results
Demographics and baseline outcome measurements
The demographics and outcome measurements at baseline were comparable between two trials (Table 1). Among 162 patients (81 females and 81 males) in STUDY 1 and 39 patients (39 females) in the STUDY 2, no significant difference in the mean of age (26.13 vs 25.54 years), and the duration of illness (8.64 vs 9.28 months) were found between STUDY 1 and STUDY 2. Although not observing any significant difference in body mass index, fasting glucose, insulin, insulin resistance index, HDL-C, LDL-C (P>0.05) at baseline, a significant difference in total cholesterol (5.22 vs 4.81 mmol l–1, P<0.0001) and triglyceride (2.40 vs 1.79 mmol l–1, P=0.0286) was noted between STUDY 1 and STUDY 2. Therefore, study was coded as a binary variable, which was then used as a control variable in the combined data analysis, in particular for these two outcomes that showed difference between treatment and placebo group.
The demographics and outcome measurements at baseline between metformin and placebo group were examined in each individual study and in the combined data. The distribution of antipsychotics used in patients with schizophrenia were 47.8% for olanzapine, 27.4% for risperidone, 14.3% for clozapine and 10.4% for sulpiride; and there were no significant difference between metformin treatment and placebo group (P=0.5104) (Supplementary Table S1). In STUDY 1, a difference in total cholesterol between metformin treatment (mean=5.69 mmol l–1) and placebo group (mean=4.78 mmol l–1) was founded (P<0.0001); whereas in STUDY 2, a differences in body weight (P=0.016), HDL-C (P=0.030) and triglyceride (P=0.043) between metformin treatment and placebo group were noted (Supplementary Table S2). When two data sets from STUDY 1 and STUDY 2 were combined, only a difference in total cholesterol remained, and it became smaller. The mean of total cholesterol was 5.41 mmol l–1 in metformin group, greater than the cutoff defining for dyslipidemia (5.18 mmol l–1), and also higher than the mean of 4.86 mmol l–1 in placebo group.
The percentage of dyslipidemia defined by four outcome measurements were different between metformin and placebo, and between two trials. However, the differences were diminished in the combined data set (Table 2). In STUDY 1, the percentage of dyslipidemia defined by all four measurements were higher in the metformin group than the placebo group; and they were significantly different in that defined by total cholesterol (65.4% vs 29.8%, P=8.44x10−06) and triglyceride (93.6% vs 72.6%, P=3.76x10−04). Although in STUDY 2, the percentage of dyslipidemia defined by total cholesterol, triglyceride and HDL-C were higher in placebo than metformin group. However, they were not statistically significant except total cholesterol, likely due to a smaller sample size. When two data sets were combined, the percentage of dyslipidemia was only significant in that defined by total cholesterol between metformin and placebo group (55.3% vs 33.7%, P=0.002). This was consistent with the difference in the mean of total cholesterol between metformin and placebo group.
Within-group trend and treatment effect
Table 3 presents the mean of all outcome measurements over the time period of treatment between metformin and placebo group in the combined data of STUDY 1 and STUDY 2. In total, there were 201 patients, 103 of them treated with metformin and 98 with placebo. During the course of treatment, all patients had a good medication adherence. Only 1 of 103 patients in the metformin group, and 3 of 98 patients in the placebo group discontinued before week 12 because of loss to follow-up, and 7 patients (3 metformin and 4 placebo) discontinued before week 24 because of loss to follow-up. Patients and their caregivers reported that 86–100% of patients in both groups had >80% of their medications.
Descriptive analysis showed that two treatment groups tend to have different trends within group. Schizophrenia patients treated with metformin showed a decreased trend in the level of insulin and lipids over time. After medication, the level of insulin was reduced from 26.15 mIU l–1 at baseline to 22.72 mIU l–1 at week 24 (P<0.0001), insulin resistance index from 6.31 to 5.45 (P<0.0001), LDL-C from 3.69 to 3.02 mmol l–1 (P<0.0001) and total cholesterol from 5.41 to 4.86 mmol l–1 (P<0.0001). In contrast, schizophrenia patients treated with placebo appears to have an increased body weight (P=0.0028), body mass index (P=0.0015) and total cholesterol (P=0.0004).
Compared with the placebo, metformin treatment have a significant effect on the level of insulin after adjusting for sex, age, duration of illness, type of medication and study cohort. Analysis of data using general linear mixed model with treatment and time interaction showed that metformin treatment effects on insulin level changed significantly over time (Table 4). Schizophrenia patients treated with metformin tend to reduce body weight, body mass index, insulin and insulin resistance index. At baseline, there is no difference in all these outcome measurements between metformin and placebo group. Metformin treatment had significant effect on the mean difference in body weight from −0.60 kg at baseline to –2.1 kg at week 24 (P=0.0007), body mass index from −0.33 to −0.89 kg m–1 (P=0.0016), insulin from –1.59 to–7.51 mIU l–1 (P<0.0001) and insulin resistance index from –0.23 to –1.64 (P<0.0001). It is interesting to note that these effects appear at week 12, and further continued at week 24. However, we did not observe any significant effect of metformin treatment on fasting glucose (P=0.3253).
We found that metformin treatment had significant effects on reducing dyslipidemia. After treatment, the mean difference in LDL-C between treatment and placebo group changed from 0.16 mmol l–1 at baseline to –0.86 mmol l–1 at the end of week 24, an decrease by 1.02 mmol l–1 because of the metformin treatment (P<0.0001); the mean difference in total cholesterol was from 0.70 to –0.49 mmol l–1, decreased by 1.19 mmol l–1 (P<0.0001); and 0.11 to –0.37 mmol l–1 in triglyceride, deceased by 0.48 mmol l–1 (P=0.0158). In contrast, the mean difference in HDL-C increased from 0.05 mmol l–1 at baseline to 0.14 mmol l–1 at week 24 (P=0.0364). It is interesting to note that except HDL-C, all these treatment effects on lipid level did not appear until the end of week 24.
In term of dyslipidemia measured by LDL-C as a binary outcome, the overall treatment effect was still significant among 190 patients (99 metformin and 91 placebo) who completed the trials. At the end of trials, only 25.3% (25/99) of patients in the metformin group had dyslipidemia defined by LDL-C (⩾3.37 mmol l–1 or 130 mg dl–1), compared with 64.8% (59/91) in the placebo group (χ2=30.12, df =1, P<0.001). For those who had dyslipidemia by LDL-C level at baseline, 64.0% (32/50) of patients who took metformin restored their LDL-C to below the normal level, compared with 23.3% (10/43) who took placebo (χ2=15.49, df =1, P<0.001). In contrast, in the placebo group, for those patients who had a normal LDL-C at baseline, 54.2% of them (26/48) had an increase in their LDL-C level to ⩾3.37 mmol l–1 (130 mg dl–1), compared with 14.3% (7/49) taking metformin (χ2=17.18, df=1, P<0.001). We did not perform multiple regression analysis of the binary outcome because of limited sample size.
Insulin resistance on metformin treatment effect
Suspecting that the effect of metformin treatment on dyslipidemia may act through the improvement of insulin resistance, we examined further treatment effect on dyslipidemua while controlling for the level of insulin resistance, measured by insulin resistance index. At baseline, correlation analysis of outcome measurements showed that body weight, body mass index, fasting glucose, insulin and insulin resistance index were moderately correlated and clustered together (r>0.30). LDL-C and total cholesterol were correlated, but HDL-C and triglyceride appear independent of all other outcome measurements (Supplementary Table S4).
Insulin resistance appears to have little impact on the improvement of dyslipidemia after metformin treatment (Table 5). When insulin resistance index was included as a covariate in the multiple regression analysis, the effect of metformin treatment on HDL-C became nonsignificant (P=0.1325). In contrast, after controlling for insulin resistance index, metformin treatment still have significant effect on LDL-C (P<0.0001), total cholesterol (P<0.0001) and triglyceride (P=0.0191). When the analysis was stratified by sex, we found that metformin treatment had significant effect on LDL-C (P<0.0001), total cholesterol (P=0.0001) and triglycerides (P=0.0027) in men; only significant effects on LDL-C (P<0.0001) and total cholesterol (P=0.0014) were observed in women, which are largely consistent between men and women. Post-hoc analysis showed that the metformin treatment effects only occurred significantly at week 24. These suggest that metformin treatment on the improvement of LDL-C, total cholesterol and triglyceride is independent from insulin resistance.
Adverse events
There were no significant differences in the frequency and types of adverse events reported between the two groups. Adverse events that affected >5% of the overall sample were showed at Supplementary Table S5. No hypoglycemia was reported during the trial. Serum lactic acid levels, liver and renal function tests, and electrocardiogram results remained normal in all patients throughout the course of the treatment, and there were no episodes of vomiting or lactic acidosis.
Discussion
To our knowledge, this is the first clinical trial that examines the effect of metformin treatment on antipsychotic-induced dyslipidemia in patients with first-episode schizophrenia. After a 24-week trial, we found that metformin treatment had significant effect on not only controlling weight gain, insulin and insulin resistance, which is consistent with our previous studies,5, 14, 15, 16 but also significantly improving the altered level of lipids, including LDL-C, HDL-C, total cholesterol and triglycerides in blood. These effects on insulin and lipids were in a time-sequence manner, and improvement of lipid profile was at least partly independent of reducing insulin resistance.
Our findings that metformin treatment decrease antipsychotic-induced elevation of LDL-C levels are consistent with previous studies in patients with diabetes24, 33and schizophrenia patients,34 as well as an animal study that showed treatment effect on triglyceride and total cholesterol.23 In study of schizophrenia patients under prolonged clozapine administration, Carrizo et al.34 found that metformin treatment have significant effect on reducing elevated level of insulin and the ratio of insulin to HDL-C, as well as improving the level of HDL-C. In a recent study, Xu et al.35 examined a large cohort for the effects of metformin on metabolite profile and LDL-C in patients with type 2 diabetes, and found that lower concentrations of three metabolites all are associated with LDL-C in blood sample. However, in a multi-center, double-blind, placebo-controlled trial using metformin to reduce body weight gain in schizophrenia or bipolar patients treated with olanzapine, Baptist et al.36 reported that metformin could improve weight gain, but not lipid profile. This inconsistency may be due to several other factors such as the heterogeneity in patients because of a multi-center study design and shorter treatment period (12 weeks).
The effects of metformin treatment on reducing insulin or insulin resistance, and dyslipidemia occurred in a time-sequence manner. We observed that metformin treatment effects on weight gain and insulin resistance were significant at week 12, which is also the same for HDL-C. However, its effect on lipid profile such as LDL-C, total cholesterol and triglycerides did not appear until the end of trial at week 24. This is interesting, and suggests that metformin treatment effect on LDL-C, total cholesterol and triglycerides appear after the decrease in insulin resistance. That may be one of the reasons to explain why the previous study with 12-week trial did not find a significant effect of metformin treatment on LDL-C level.36 There have been evidences showing that high insulin resistance is associated with increased cholesterol synthesis and decreased absorption in high-risk vascular patients who were not on lipid-lowering therapy37 or in normoglycemic men.38 This may explain that both insulin resistance and dyslipidemia are believed to be risk factors for cardiovascular disease.39, 40 That metformin treatment improves insulin resistance and lipid profile may provide an evidence for an approximate causal relationship between reducing insulin resistance and improving lipid profile in schizophrenia patients treated with antipsychotics.
The evidence that metformin treatment improve the lipid profile in schizophrenia patients is to a certain degree independent of insulin resistance. After controlling for insulin resistance index in the regression model, metformin treatment still have significant effect on reducing the level of LDL-C, total cholesterol and triglycerides, although it is known that the metformin treatment affect insulin resistance, which high level of insulin resistance index has been associated with increased cholesterol synthesis precursors and decreased absorption markers.37, 38 In contrast, metformin treatment effect on improvement of HDL-C was mediated through the insulin resistance, as that after we controlled for insulin resistance index in the analysis, treatment effect was no longer significant. We should point out that among four lipid measurements, HDL-C were independent of LDL-C, total cholesterol and triglyceride. However, we did not observe metformin treatment effect on fasting glucose.
Our study showed that the reduced insulin resistance explain the improvement of HDL-C, but may not fully explain its effect on other lipids such as LDL-C, total cholesterol and triglyceride. The molecular mechanisms underlying metformin action appear related to its activation of the energy sensor AMP-activated protein kinase.41, 42 Metformin is transported into hepatocytes mainly through organic cation transporter 1, leading to inhibit mitochondrial respiration chain (complex I), and then causing a decrease in energy availability in cell. The decrease in energy availability is balanced by reducing energy consumption, in particularly reduced gluconcogenesis in the liver. This is mediated through signaling and metabolic flux effects that results from (1) a decrease in ATP and increase in AMP concentration, which directly contribute to the inhibition of gluconcogenesis, and (2) the increased AMP levels function as a key signaling mediator that inhibits (cyclic adenosine 3',5'-monophosphate protein kinase A and FBPase, but activates AMP-activated protein kinase. As a result, inhibition of cyclic adenosine 3',5'-monophosphate protein kinase A and FBPase lead to decrease in glucose production; whereas activation of AMP-activated protein kinase causes a decrease in lipid and cholesterol synthesis.41 This mechanism may explain that metformin treatment can attenuate the altered level of both insulin resistance and lipid independently. A recent analysis of genomics and metabolomic data provided an empirical support in humans that reduced levels in three metabolites (acyl-alkyl phosphatidylcholines) are consistently lower in type 2 diabetes mellitus patients treated with metformin, and they all were also associated with lowered blood level of LDL-C. The variations in these three metabolites were significantly associated with genetic variants at 17 genes including FADS1 and FADS2, which are controlled by AMP-activated protein kinase.35
Possible limitations
Our study should be read with some limitations. First, this study was based on schizophrenia patients treated with four different antipsychotic drugs including clozapine, olanzapine, risperidone and sulpiride. Previous study has shown that overall the type of medications affect the total cholesterol, triglyceride and prolactin significantly.43 Unfortunately, we were unable to assess this effect by type of medication because of the smaller sample size in our study. Second, we did not monitor the level of prolactin, which is one of adverse effects induced by antipsychotics.44 As most of typical and atypical antipsychotic medications block the dopamine D2 receptor to help reducing the excess level of dopamine, which prevents excess prolactin secretion from the pituitary gland. Antipsychotic medication may lead to elevated level of prolactin in plasma.45 In addition, we did not monitor serum B12 and folate levels, although our laboratory tests did indicate that there was no decline in hemoglobin levels in any of the patients. Finally, we did not examine pharmacokinetic interactions between antipsychotics and metformin, nor monitor drug clearance. Metformin is unlikely to have interactions with antipsychotics because it is not metabolized and does not inhibit the metabolism of other drugs. Evidence has shown that slower metabolizer of risperidone may have a better treatment response in symptoms.31 This may affect antipsychotic drug response at least for risperidone.
In conclusion, despite these limitations, this study has shown clearly that the addition of metformin to antipsychotics is a potential treatment to attenuate dyslipidemia in patients with schizophrenia.
References
De Hert M, Detraux J, van Winkel R, Yu W, Correll CU . Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2011; 8: 114–126.
Heal DJ, Gosden J, Jackson HC, Cheetham SC, Smith SL . Metabolic consequences of antipsychotic therapy: preclinical and clinical perspectives on diabetes, diabetic ketoacidosis, and obesity. Handb Exp Pharmacol 2012; 212: 135–164.
Ruzanna ZZ, Ong LY, Cheah YC, Fairuz A, Marhani M . The association between dyslipidaemia and types of antipsychotic medications among patients with chronic schizophrenia. Med J Malaysia 2012; 67: 39–44.
Musil R, Obermeier M, Russ P, Hamerle M . Weight gain and antipsychotics: a drug safety review. Expert Opin Drug Saf 2015; 14: 73–96.
Wu RR, Zhao JJ, Liu ZN, Zhai JG, Guo XF, Guo WB et al. Effects of typical and atypical antipsychotics on glucose-insulin homeostasis and lipid metabolism in first-episode schizophrenia. Psychopharmacology (Berl) 2006; 186: 572–578.
Graham I, Cooney MT, Bradley D, Dudina A, Reiner Z . Dyslipidemias in the prevention of cardiovascular disease: risks and causality. Curr Cardiol Rep 2012; 14: 709–720.
Casey DE . Metabolic issues and cardiovascular diseases in patients with psychiatric disorders. Am J Med 2005; 118: 15s–22s.
Lambert TJ, Velakoulis D, Pantelis C . Medical comorbidity in schizophrenia. Med J Aust 2003; 178: S67–S70.
Jarcho JA, Keaney JF Jr . Proof that lower is better—LDL cholesterol and IMPROVE-IT. N Engl J Med 2015; 372: 2448–2450.
Chinese Cardiovascular Disease Association The Guidelines of Dyslipidemia Prevention in China. People Health Press: Beijing, China, 2007.
Jellinger PS, Smith DA, Mehta AE, Ganda O, Handelsman Y, Rodbard HW et al. American Association of Clinical Endocrinologists' Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis: executive summary. Endocr Pract 2012; 18: 269–293.
Jellinger PS, Smith DA, Mehta AE, Ganda O, Handelsman Y, Rodbard HW et al. American Association of Clinical Endocrinologists' Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocr Pract 2012; 18: 1–78.
Izkhakov E, Meltzer E, Rubinstein A . Pathogenesis and management of diabetic dyslipidemia. Treat Endocrinol 2003; 2: 231–245.
Wu RR, Jin H, Gao K, Twamley EW, Ou JJ, Shao P et al. Metformin for treatment of antipsychotic-induced amenorrhea and weight gain in women with first-episode schizophrenia: a double-blind, randomized, placebo-controlled study. Am J Psychiatry 2012; 169: 813–821.
Wu RR, Zhao JP, Guo XF, He YQ, Fang MS, Guo WB et al. Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled study. Am J Psychiatry 2008; 165: 352–358.
Wu RR, Zhao JP, Jin H, Shao P, Fang MS, Guo XF et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA 2008; 299: 185–193.
Grundy SM . Atherogenic dyslipidemia associated with metabolic syndrome and insulin resistance. Clin Cornerstone 2006; 8: S21–S27.
Soleimani M . Insulin resistance and hypertension: new insights. Kidney Int 2015; 87: 497–499.
Bornfeldt KE, Tabas I . Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab 2011; 14: 575–585.
Avramoglu RK, Basciano H, Adeli K . Lipid and lipoprotein dysregulation in insulin resistant states. Clin Chim Acta 2006; 368: 1–19.
Taghibiglou C, Carpentier A, Van Iderstine SC, Chen B, Rudy D, Aiton A et al. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. Evidence for enhanced lipoprotein assembly, reduced intracellular ApoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J Biol Chem 2000; 275: 8416–8425.
Toth PP . Insulin resistance, small LDL particles, and risk for atherosclerotic disease. Curr Vasc Pharmacol 2014; 12: 653–657.
Adeneye AA, Agbaje EO, Olagunju JA . Metformin: an effective attenuator of risperidone-induced insulin resistance hyperglycemia and dyslipidemia in rats. Indian J Exp Biol 2011; 49: 332–338.
Robinson AC, Burke J, Robinson S, Johnston DG, Elkeles RS . The effects of metformin on glycemic control and serum lipids in insulin-treated NIDDM patients with suboptimal metabolic control. Diabetes Care 1998; 21: 701–705.
American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. American Psychiatric Association: Washington, DC, USA, 1994.
First MB, Spitzer RL, Gibbon M, Williams JBW . Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV). American Psychiatric Press: Washington, DC, USA, 1996.
Ji LN, Pan CY, Lu JM, Li H, Li Q, Li QF et al. Efficacy and safety of combination therapy with vildagliptin and metformin versus metformin up-titration in Chinese patients with type 2 diabetes mellitus: study design and rationale of the vision study. Cardiovasc Diabetol 2013; 12: 118.
Haffner SM, Miettinen H, Stern MP . The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997; 20: 1087–1092.
Andreasen NC . Scale for Assessment of Negative and Positive Symptoms. University of Iowa Press: Iowa City, USA, 1984.
Guy W . ECDEU Assessment Manual for Psychopharmacology, Revised. US Dept of Health, Education, and Welfare: Rockville, MD, USA, 1976; 341–350.
Heide J, Zhang F, Bigos KL, Mann SA, Carr VJ, Shannon Weickert C et al. Differential response to risperidone in schizophrenia patients by KCNH2 genotype and drug metabolizer status. Am J Psychiatry 2016; 173: 53–59.
Apud JA, Zhang F, Decot H, Bigos KL, Weinberger DR . Genetic variation in KCNH2 associated with expression in the brain of a unique hERG isoform modulates treatment response in patients with schizophrenia. Am J Psychiatry 2012; 169: 725–734.
Mullugeta Y, Chawla R, Kebede T, Worku Y . Dyslipidemia associated with poor glycemic control in type 2 diabetes mellitus and the protective effect of metformin supplementation. Indian J Clin Biochem 2012; 27: 363–369.
Carrizo E, Fernández V, Connell L, Sandia I, Prieto D, Mogollón J et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophr Res 2009; 113: 19–26.
Xu T, Brandmaier S, Messias AC, Herder C, Draisma HH, Demirkan A et al. Effects of metformin on metabolite profiles and LDL cholesterol in patients with type 2 diabetes. Diabetes Care 2015; 38: 1858–1867.
Baptista T, Rangel N, Fernández V, Carrizo E, El Fakih Y, Uzcátegui E et al. Metformin as an adjunctive treatment to control body weight and metabolic dysfunction during olanzapine administration: a multicentric, double-blind, placebo-controlled trial. Schizophr Res 2007; 93: 99–108.
Hoenig MR, Sellke FW . Insulin resistance is associated with increased cholesterol synthesis, decreased cholesterol absorption and enhanced lipid response to statin therapy. Atherosclerosis 2010; 211: 260–265.
Pihlajamaki J, Gylling H, Miettinen TA, Laakso M . Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglyc emic men. J Lipid Res 2004; 45: 507–512.
Ginsberg HN . Insulin resistance and cardiovascular disease. J Clin Invest 2000; 106: 453–458.
Ginsberg HN . Risk factors common to insulin resistance and atherosclerosis explain why diabetes is a cardiovascular disease. Am J Manag Care 2002; 3 Suppl: 5.
Rena G, Pearson ER, Sakamoto K . Molecular mechanism of action of metformin: old or new insights? Diabetologia 2013; 56: 1898–1906.
Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest 2007; 117: 1422–1431.
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353: 1209–1223.
Henderson DC, Doraiswamy PM . Prolactin-related and metabolic adverse effects of atypical antipsychotic agents. J Clin Psychiatry 2008; 69: 32–44.
Bargiota SI, Bonotis KS, Messinis IE, Angelopoulos NV . The effects of antipsychotics on prolactin levels and women's menstruation. Schizophr Res Treatment 2013; 2013: 502697.
Acknowledgements
The research was supported by the National Natural Science Foundation of China (grant no.81371481 to R-RW) and National Science and Technology major projects (grant no. 2012ZZX09303014-001 to J-PZ).
Author contributions
J-P Zhao had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: R-R Wu and J-P Zhao. Acquisition of data: R-R Wu, J-P Zhao, J-J Ou and P Shao. Analysis and interpretation of data: R-R Wu and F-Y Zhang. Drafting of the manuscript: R-R Wu, F-Y Zhang and K-M Gao. Critical revision of the manuscript for important intellectual content: R-R Wu, J-P Zhao, F-Y Zhang, K-M Gao, J-J Ou, H Jin and P Shao. Statistical analysis: R-R Wu, F-Y Zhang and PK Chan. Obtained funding: R-R Wu. Administrative, technical or material support: R-R Wu, J-P Zhao, J-J Ou and P Shao. Study supervision: R-R Wu and J-P Zhao.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Wu, RR., Zhang, FY., Gao, KM. et al. Metformin treatment of antipsychotic-induced dyslipidemia: an analysis of two randomized, placebo-controlled trials. Mol Psychiatry 21, 1537–1544 (2016). https://doi.org/10.1038/mp.2015.221
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.221
This article is cited by
-
Metformin improves cognitive impairment in patients with schizophrenia: associated with enhanced functional connectivity of dorsolateral prefrontal cortex
Translational Psychiatry (2023)
-
Targeted metabolomics reveals aberrant profiles of serum bile acids in patients with schizophrenia
Schizophrenia (2022)
-
PCSK9 mediates dyslipidemia induced by olanzapine treatment in schizophrenia patients
Psychopharmacology (2022)
-
Coadministration of metformin prevents olanzapine-induced metabolic dysfunction and regulates the gut-liver axis in rats
Psychopharmacology (2021)
-
Adjunctive metformin for antipsychotic-induced dyslipidemia: a meta-analysis of randomized, double-blind, placebo-controlled trials
Translational Psychiatry (2020)