Abstract
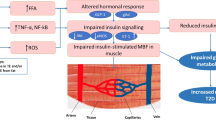
Resistance to the actions of insulin is strongly associated with the microvascular complications of diabetes. To the extent that insulin resistance leads to hyperglycemia, dyslipidemia and hypertension, this association is not surprising. It is now clear that insulin also has direct actions in the microvasculature that influence the development and progression of microvascular disease. In the healthy state, insulin appears to have only minor effects on vascular function, because of the activation of opposing mediators such as nitric oxide and endothelin-1. Diabetes and obesity, however, are associated with selective insulin resistance in the phosphatidylinositol-3-kinase signaling pathway, which leads to reduced synthesis of nitric oxide, impaired metabolic control and compensatory hyperinsulinemia. By contrast, insulin signaling via extracellular signal-regulated kinase dependent pathways is relatively unaffected in diabetes, tipping the balance of insulin's actions so that they favor abnormal vasoreactivity, angiogenesis, and other pathways implicated in microvascular complications and hypertension. In addition, preferential impairment of nonoxidative glucose metabolism leads to increased intracellular formation of advanced glycation end products, oxidative stress and activation of other pathogenic mediators. Despite a strong temporal association, a causal link between pathway-selective insulin resistance and microvascular damage remains to be established. It is possible that this association reflects a common genotype or phenotype. Nonetheless, insulin resistance remains an important marker of risk and a key target for intervention, because those patients who achieve a greater improvement of insulin sensitivity achieve better microvascular outcomes.
Key Points
-
Resistance to the actions of insulin is strongly associated with the microvascular complications of diabetes, independently of metabolic control and hypertension
-
Insulin resistance is an important marker of risk and a key target for intervention, as those patients who achieve a greater improvement of insulin sensitivity achieve better microvascular outcomes
-
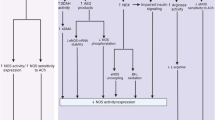
Diabetes and obesity are associated with pathway-selective insulin resistance in the phosphatidylinositol-3-kinase signaling pathway, while signaling via extracellular signal-regulated kinase dependent pathways is comparatively unaffected, tipping the balance of insulin's actions in favor of abnormal vasoreactivity, angiogenesis, and other pathways implicated in microangiopathy
-
Insulin resistance is able to enhance key pathways involved in hyperglycemia-induced microvascular damage and to exacerbate hypertension
-
The strong association between insulin resistance and microvascular disease might also reflect a common genotype or phenotype
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wild S et al. (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053
De Jager J et al. (2005) Effects of short-term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med 257: 100–109
Guan Y (2004) Peroxisome proliferator-activated receptor family and its relationship to renal complications of the metabolic syndrome. J Am Soc Nephrol 15: 2801–2815
Glass AR et al. (1981) Normal valine disposal in obese subjects with impaired glucose disposal: evidence for selective insulin resistance. Metabolism 30: 578–582
Cusin I et al. (1990) Metabolic consequences of hyperinsulinaemia imposed on normal rats on glucose handling by white adipose tissue, muscles and liver. Biochem J 267: 99–103
Prada PO et al. (2005) Western diet modulates insulin signaling, c-Jun N-terminal kinase activity, and insulin receptor substrate-1ser307 phosphorylation in a tissue-specific fashion. Endocrinology 146: 1576–1587
Cusi K et al. (2000) Insulin resistance differentially affects the PI3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest 105: 311–320
Groop L et al. (1993) Insulin resistance, hypertension and microalbuminuria in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 36: 642–647
Stumvoll M et al. (2005) Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365: 1333–1346
Lazar DF et al. (1995) Mitogen-activated protein kinase kinase inhibition does not block the stimulation of glucose utilization by insulin. J Biol Chem 270: 20801–20807
Goalstone M et al. (1997) Insulin stimulates the phosphorylation and activity of farnesyltransferase via the Ras-mitogen-activated protein kinase pathway. Endocrinology 138: 5119–5124
Rommel C et al. (1999) Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 286: 1738–1741
Patti ME et al. (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100: 8466–8471
Shulman GI (2000) Cellular mechanisms of insulin resistance. J Clin Invest 106: 171–176
Ozcan U et al. (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461
Nakamura M et al. (2001) Excessive hexosamines block the neuroprotective effect of insulin and induce apoptosis in retinal neurons. J Biol Chem 276: 43748–43755
Nakajima K et al. (2000) Selective attenuation of metabolic branch of insulin receptor down-signaling by high glucose in a hepatoma cell line, HepG2 cells. J Biol Chem 275: 20880–20886
Jiang ZY et al. (1999) Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest 104: 447–457
Sasaoka T et al. (1994) Evidence for a functional role of Shc proteins in mitogenic signaling induced by insulin, insulin-like growth factor-1, and epidermal growth factor. J Biol Chem 269: 13689–13694
Cleland SJ et al. (2000) Insulin action is associated with endothelial function in hypertension and type 2 diabetes. Hypertension 35: 507–511
Scherrer U and Sartori C (2000) Defective nitric oxide synthesis: a link between metabolic insulin resistance, sympathetic overactivity and cardiovascular morbidity. Eur J Endocrinol 142: 315–323
Caballero AE et al. (1999) Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes 48: 1856–1862
Vicent D et al. (2003) The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest 111: 1373–1380
Cardillo C et al. (1999) Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation 100: 820–825
Irving RJ et al. (2001) Activation of the endothelin system in insulin resistance. QJM 94: 321–326
Tooke JE (1995) Microvascular function in human diabetes. A physiological perspective. Diabetes 44: 721–726
Muller-Wieland D et al. (1998) Metabolic syndrome and hypertension: pathophysiology and molecular basis of insulin resistance. Basic Res Cardiol 93 (Suppl 2): 131–134
Thorn LM et al. (2005) Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care 28: 2019–2024
Yu Y et al. (2004) Insulin resistance and endothelial dysfunction in type 2 diabetes patients with or without microalbuminuria. Diabetes Res Clin Pract 65: 95–104
Wiggam MI et al. (2001) Insulin action and skeletal muscle blood flow in patients with Type 1 diabetes and microalbuminuria. Diabetes Res Clin Pract 53: 73–83
Ekstrand AV et al. (1998) Insulin resistance precedes microalbuminuria in patients with insulin-dependent diabetes mellitus. Nephrol Dial Transplant 13: 3079–3083
De Cosmo S et al. (2005) Increased urinary albumin excretion, insulin resistance, and related cardiovascular risk factors in patients with type 2 diabetes: evidence of a sex-specific association. Diabetes Care 28: 910–915
Nestler JE et al. (1990) Increased transcapillary escape rate of albumin in nondiabetic men in response to hyperinsulinemia. Diabetes 39: 1212–1217
Norgaard K et al. (1991) Effects of insulin on renal haemodynamics and sodium handling in normal subjects. Scand J Clin Lab Invest 51: 367–376
Dengel DR et al. (1996) Insulin resistance, elevated glomerular filtration fraction, and renal injury. Hypertension 28: 127–132
Toyoda M et al. (2004) High expression of PKC-MAPK pathway mRNAs correlates with glomerular lesions in human diabetic nephropathy. Kidney Int 66: 1107–1114
Hasan RN et al. (2003) Differential regulation of early growth response gene-1 expression by insulin and glucose in vascular endothelial cells. Arterioscler Thromb Vasc Biol 23: 988–993
Chaturvedi N et al. (2001) Markers of insulin resistance are strong risk factors for retinopathy incidence in type 1 diabetes. Diabetes Care 24: 284–289
Parvanova A et al. (2004) Insulin resistance and proliferative retinopathy: a cross-sectional, case-control study in 115 patients with type 2 diabetes. J Clin Endocrinol Metab 89: 4371–4376
Hadjadj S et al. (2004) Different patterns of insulin resistance in relatives of type 1 diabetic patients with retinopathy or nephropathy: the Genesis France-Belgium Study. Diabetes Care 27: 2661–2668
Gabir MM et al. (2000) Plasma glucose and prediction of microvascular disease and mortality: evaluation of 1997 American Diabetes Association and 1999 World Health Organization criteria for diagnosis of diabetes. Diabetes Care 23: 1113–1118
Abiko T et al. (2003) Characterization of retinal leukostasis and hemodynamics in insulin resistance and diabetes: role of oxidants and protein kinase-C activation. Diabetes 52: 829–837
Chou E et al. (2002) Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic states: a possible explanation for impaired collateral formation in cardiac tissue. Circulation 105: 373–379
Doronzo G et al. (2004) Insulin activates vascular endothelial growth factor in vascular smooth muscle cells: influence of nitric oxide and of insulin resistance. Eur J Clin Invest 34: 664–673
Lee RH and Dellon AL (1999) Insulin resistance. Does it play a role in peripheral neuropathy? Diabetes Care 22: 1914–1915
Sumner CJ et al. (2003) The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 60: 108–111
Thrainsdottir S et al. (2003) Endoneurial capillary abnormalities presage deterioration of glucose tolerance and accompany peripheral neuropathy in man. Diabetes 52: 2615–2622
Sugimoto K et al. (2003) Peripheral neuropathy and microangiopathy in rats with insulinoma: association with chronic hyperinsulinemia. Diabetes Metab Res Rev 19: 392–400
Tesfaye S et al. (1996) Arterio-venous shunting and proliferating new vessels in acute painful neuropathy of rapid glycaemic control (insulin neuritis). Diabetologia 39: 329–335
Brussee V et al. (2004) Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes 53: 1824–1830
Huang TJ et al. (2005) Insulin enhances mitochondrial inner membrane potential and increases ATP levels through phosphoinositide 3-kinase in adult sensory neurons. Mol Cell Neurosci 28: 42–54
Ogata T et al. (2004) Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J Neurosci 24: 6724–6732
Ferrannini E et al. (1987) Insulin resistance in essential hypertension. N Engl J Med 317: 350–357
Vedovato M et al. (2004) Effect of sodium intake on blood pressure and albuminuria in Type 2 diabetic patients: the role of insulin resistance. Diabetologia 47: 300–303
Ter Maaten JC et al. (1997) Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin Sci (Lond) 92: 51–58
Hall JE et al. (1995) Hemodynamic and renal responses to chronic hyperinsulinemia in obese, insulin-resistant dogs. Hypertension 25: 994–1002
Ter Maaten JC et al. (1999) Insulin's acute effects on glomerular filtration rate correlate with insulin sensitivity whereas insulin's acute effects on proximal tubular sodium reabsorption correlation with salt sensitivity in normal subjects. Nephrol Dial Transplant 14: 2357–2363
Gans RO et al. (1996) The renal response to exogenous insulin in non-insulin-dependent diabetes mellitus in relation to blood pressure and cardiovascular hormonal status. Nephrol Dial Transplant 11: 794–802
Zhang SL et al. (2002) Insulin inhibits dexamethasone effect on angiotensinogen gene expression and induction of hypertrophy in rat kidney proximal tubular cells in high glucose. Endocrinology 143: 4627–4635
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820
Kelley DE et al. (2002) Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950
Al-Khalili L et al. (2005) Enhanced insulin-stimulated glycogen synthesis in response to insulin, metformin or rosiglitazone is associated with increased mRNA expression of GLUT4 and peroxisomal proliferator activator receptor γ co-activator 1. Diabetologia 48: 1173–1179
Itani SI et al. (2002) Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 51: 2005–2011
Bell KS et al. (2000) Acute reversal of lipid-induced muscle insulin resistance is associated with rapid alteration in PKC-θ localization. Am J Physiol Endocrinol Metab 279: 1196–1201
Miele C et al. (2003) Human glycated albumin affects glucose metabolism in L6 skeletal muscle cells by impairing insulin-induced insulin receptor substrate (IRS) signaling through a protein kinase C α-mediated mechanism. J Biol Chem 278: 47376–47387
Hofmann SM et al. (2002) Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes 51: 2082–2089
Rahbar S et al. (2000) Evidence that pioglitazone, metformin and pentoxifylline are inhibitors of glycation. Clin Chim Acta 301: 65–77
Wang J et al. (1998) A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 393: 684–688
Pouwels MJ et al. (2004) Role of hexosamines in insulin resistance and nutrient sensing in human adipose and muscle tissue. J Clin Endocrinol Metab 89: 5132–5137
Yasunari K et al. (2000) Aldose reductase inhibitor improves insulin-mediated glucose uptake and prevents migration of human coronary artery smooth muscle cells induced by high glucose. Hypertension 35: 1092–1098
Cook S et al. (2004) Partial gene deletion of endothelial nitric oxide synthase predisposes to exaggerated high-fat diet-induced insulin resistance and arterial hypertension. Diabetes 53: 2067–2072
Zanchi A et al. (2000) Risk of advanced diabetic nephropathy in type 1 diabetes is associated with endothelial nitric oxide synthase gene polymorphism. Kidney Int 57: 405–413
Book CB and Dunaif A (1999) Selective insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab 84: 3110–3116
Paradisi G et al. (2001) Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation 103: 1410–1415
Yajnik CS et al. (1995) Fetal growth and glucose and insulin metabolism in four-year-old Indian children. Diabet Med 12: 330–336
Fagerudd J et al. (2004) Birth weight is inversely correlated to adult systolic blood pressure and pulse pressure in type 1 diabetes. Hypertension 44: 832–837
Newman WP and Brodows RG (1983) Insulin action during acute starvation: evidence for selective insulin resistance in normal man. Metabolism 32: 590–596
Amiel SA et al. (1991) Insulin resistance of puberty: a defect restricted to peripheral glucose metabolism. J Clin Endocrinol Metab 72: 277–282
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Groop, PH., Forsblom, C. & Thomas, M. Mechanisms of Disease: pathway-selective insulin resistance and microvascular complications of diabetes. Nat Rev Endocrinol 1, 100–110 (2005). https://doi.org/10.1038/ncpendmet0046
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0046
This article is cited by
-
Biomarkers and signaling pathways of diabetic nephropathy and peripheral neuropathy: possible therapeutic intervention of rutin and quercetin
Diabetology International (2023)
-
Association of metabolic syndrome traits with urinary biomarkers in Japanese adults
Diabetology & Metabolic Syndrome (2022)
-
Comprehensive elaboration of glycemic variability in diabetic macrovascular and microvascular complications
Cardiovascular Diabetology (2021)
-
Diabetische Nephropathie bei den neuen Diabetessubphänotypen
Der Diabetologe (2021)
-
Inhibition of tumor necrosis factor-α enhanced the antifibrotic effect of empagliflozin in an animal model with renal insulin resistance
Molecular and Cellular Biochemistry (2020)