Abstract
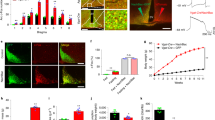
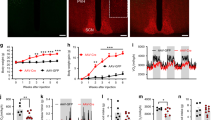
The hypothalamus responds to circulating leptin and insulin in the control of food intake and body weight. A number of neurotransmitters in the hypothalamus, including γ-aminobutyric acid (GABA), also have key roles in feeding. Huntingtin-associated protein 1 (Hap1) is expressed more abundantly in the hypothalamus than in other brain regions, and lack of Hap1 in mice leads to early postnatal death. Hap1 is also involved in intracellular trafficking of the GABAA receptor. Here, we report that fasting upregulates the expression of Hap1 in the rodent hypothalamus, whereas intracerebroventricular administration of insulin downregulates Hap1 by increasing its degradation through ubiquitination. Decreasing the expression of mouse hypothalamic Hap1 by siRNA reduces the level and activity of hypothalamic GABAA receptors and causes a decrease in food intake and body weight. These findings provide evidence linking hypothalamic Hap1 to GABA in the stimulation of feeding and suggest that this mechanism is involved in the feeding-inhibitory actions of insulin in the brain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Friedman, J.M. & Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998).
Saper, C.B., Chou, T.C. & Elmquist, J.K. The need to feed: homeostatic and hedonic control of eating. Neuron 36, 199–211 (2002).
Schwartz, M.W. & Porte, D., Jr. Diabetes, obesity, and the brain. Science 307, 375–379 (2005).
Benoit, S.C., Clegg, D.J., Seeley, R.J. & Woods, S.C. Insulin and leptin as adiposity signals. Recent Prog. Horm. Res. 59, 267–285 (2004).
Kalra, S.P. et al. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 20, 68–100 (1999).
Leibowitz, S.F. & Wortley, K.E. Hypothalamic control of energy balance: different peptides, different functions. Peptides 25, 473–504 (2004).
van den Pol, A.N. Weighing the role of hypothalamic feeding neurotransmitters. Neuron 40, 1059–1061 (2003).
Olgiati, V.R., Netti, C., Guidobono, F. & Pecile, A. The central GABAergic system and control of food intake under different experimental conditions. Psychopharmacology (Berl.) 68, 163–167 (1980).
Tsujii, S. & Bray, G.A. GABA-related feeding control in genetically obese rats. Brain Res. 540, 48–54 (1991).
Stratford, T.R. & Kelley, A.E. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J. Neurosci. 17, 4434–4440 (1997).
Pu, S. et al. Interactions between neuropeptide Y and gamma-aminobutyric acid in stimulation of feeding: a morphological and pharmacological analysis. Endocrinology 140, 933–940 (1999).
Li, X.J. et al. A huntingtin-associated protein enriched in brain with implications for pathology. Nature 378, 398–402 (1995).
Li, X.J. et al. Huntingtin-associated protein (HAP1): discrete neuronal localizations in the brain resemble those of neuronal nitric oxide synthase. Proc. Natl. Acad. Sci. USA 93, 4839–4844 (1996).
Gutekunst, C.A. et al. The cellular and subcellular localization of huntingtin-associated protein 1 (HAP1): comparison with huntingtin in rat and human. J. Neurosci. 18, 7674–7686 (1998).
Page, K.J., Potter, L., Aronni, S., Everitt, B.J. & Dunnett, S.B. The expression of Huntingtin-associated protein (HAP1) mRNA in developing, adult and ageing rat CNS: implications for Huntington's disease neuropathology. Eur. J. Neurosci. 10, 1835–1845 (1998).
Fujinaga, R. et al. Neuroanatomical distribution of Huntingtin-associated protein-1 mRNA in the male mouse brain. J. Comp. Neurol. 478, 88–109 (2004).
Chan, E.Y. et al. Targeted disruption of Huntingtin-associated protein-1 (Hap1) results in postnatal death due to depressed feeding behavior. Hum. Mol. Genet. 11, 945–959 (2002).
Li, S.H. et al. Lack of huntingtin-associated protein-1 causes neuronal death resembling hypothalamic degeneration in Huntington's disease. J. Neurosci. 23, 6956–6964 (2003).
Dragatsis, I., Zeitlin, S. & Dietrich, P. Huntingtin-associated protein 1 (Hap1) mutant mice bypassing the early postnatal lethality are neuroanatomically normal and fertile but display growth retardation. Hum. Mol. Genet. 13, 3115–3125 (2004).
Vonsattel, J.P. et al. Neuropathological classification of Huntington's disease. J. Neuropathol. Exp. Neurol. 44, 559–577 (1985).
Kremer, H.P., Roos, R.A., Dingjan, G., Marani, E. & Bots, G.T. Atrophy of the hypothalamic lateral tuberal nucleus in Huntington's disease. J. Neuropathol. Exp. Neurol. 49, 371–382 (1990).
Sanberg, P.R., Fibiger, H.C. & Mark, R.F. Body weight and dietary factors in Huntington's disease patients compared with matched controls. Med. J. Aust. 1, 407–409 (1981).
Pratley, R.E., Salbe, A.D., Ravussin, E. & Caviness, J.N. Higher sedentary energy expenditure in patients with Huntington's disease. Ann. Neurol. 47, 64–70 (2000).
Petersen, A. et al. Orexin loss in Huntington's disease. Hum. Mol. Genet. 14, 39–47 (2005).
Gauthier, L.R. et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell 118, 127–138 (2004).
Li, X.J. & Li, S.H. HAP1 and intracellular trafficking. Trends Pharmacol. Sci. 26, 1–3 (2005).
McGuire, J.R., Rong, J., Li, S.H. & Li, X.J. Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J. Biol. Chem. 281, 3552–3559 (2006).
Kittler, J.T. et al. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-aminobutyric acid type A receptor membrane trafficking. Proc. Natl. Acad. Sci. USA 101, 12736–12741 (2004).
MacDonald, P.E. et al. Impaired glucose-stimulated insulin secretion, enhanced intraperitoneal insulin tolerance, and increased β-cell mass in mice lacking the p110gamma isoform of phosphoinositide 3-kinase. Endocrinology 145, 4078–4083 (2004).
Chang, G.Q., Karatayev, O., Davydova, Z., Wortley, K. & Leibowitz, S.F. Glucose injection reduces neuropeptide Y and agouti-related protein expression in the arcuate nucleus: a possible physiological role in eating behavior. Brain Res. Mol. Brain Res. 135, 69–80 (2005).
Yang, X.J., Kow, L.M., Funabashi, T. & Mobbs, C.V. Hypothalamic glucose sensor: similarities to and differences from pancreatic β-cell mechanisms. Diabetes 48, 1763–1772 (1999).
O'Brien, R.M. & Granner, D.K. Regulation of gene expression by insulin. Physiol. Rev. 76, 1109–1161 (1996).
Samson, S.L. & Wong, N.C. Role of Sp1 in insulin regulation of gene expression. J. Mol. Endocrinol. 29, 265–279 (2002).
Rui, L., Fisher, T.L., Thomas, J. & White, M.F. Regulation of insulin/insulin-like growth factor-1 signaling by proteasome-mediated degradation of insulin receptor substrate-2. J. Biol. Chem. 276, 40362–40367 (2001).
Matsuzaki, H., Daitoku, H., Hatta, M., Tanaka, K. & Fukamizu, A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc. Natl. Acad. Sci. USA 100, 11285–11290 (2003).
Herring, D. et al. Constitutive GABAA receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin-binding motif within the beta 2 subunit of the receptor. J. Biol. Chem. 278, 24046–24052 (2003).
Wan, Q. et al. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature 388, 686–690 (1997).
Decavel, C. & Van den Pol, A.N. GABA: a dominant neurotransmitter in the hypothalamus. J. Comp. Neurol. 302, 1019–1037 (1990).
Li, S.H., Li, H., Torre, E.R. & Li, X.J. Expression of huntingtin-associated protein-1 in neuronal cells implicates a role in neuritic growth. Mol. Cell. Neurosci. 16, 168–183 (2000).
Coscina, D.V., Castonguay, T.W. & Stern, J.S. Effects of increasing brain GABA on the meal patterns of genetically obese vs. lean Zucker rats. Int. J. Obes. Relat. Metab. Disord. 16, 425–433 (1992).
Carvalheira, J.B. et al. Selective impairment of insulin signalling in the hypothalamus of obese Zucker rats. Diabetologia 46, 1629–1640 (2003).
Staley, K.J., Soldo, B.L. & Proctor, W.R. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science 269, 977–981 (1995).
McBain, C.J. & Fisahn, A. Interneurons unbound. Nat. Rev. Neurosci. 2, 11–23 (2001).
Emmerson, P.J. & Miller, R.J. Pre- and postsynaptic actions of opioid and orphan opioid agonists in the rat arcuate nucleus and ventromedial hypothalamus in vitro. J. Physiol. (Lond.) 517, 431–445 (1999).
Vogt, K., Mellor, J., Tong, G. & Nicoll, R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 26, 187–196 (2000).
Acknowledgements
We thank V. Jaswani for technical assistance and the members of the Li laboratory for comments and critical reading. This work was supported by US National Institutes of Health grants NS36232 and AG19206 (to X.J.L.), NS045016 (to S.H.L.), P01 HD29587 (to S.A.L.), MH43422 to (S.F.L.) and AG00975 (to G.T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Hap1 transcripts in the hypothalamus. (PDF 166 kb)
Supplementary Fig. 2
Immunostaining of the hypothalamus with Hap1-specific antibody. (PDF 105 kb)
Supplementary Fig. 3
Hypothalamic expression of Hap1 after intracerebroventricular injection of insulin, NPY or leptin. (PDF 229 kb)
Supplementary Fig. 4
Colocalization of Hap1 with GABAA β2/3 subunits. (PDF 173 kb)
Supplementary Fig. 5
Hap1 expression and other peptides in the hypothalamus. (PDF 282 kb)
Rights and permissions
About this article
Cite this article
Sheng, G., Chang, Gq., Lin, J. et al. Hypothalamic huntingtin-associated protein 1 as a mediator of feeding behavior. Nat Med 12, 526–533 (2006). https://doi.org/10.1038/nm1382
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1382
This article is cited by
-
Biological functions and potential therapeutic applications of huntingtin-associated protein 1: progress and prospects
Clinical and Translational Oncology (2022)
-
Differential expression and roles of Huntingtin and Huntingtin-associated protein 1 in the mouse and primate brains
Cellular and Molecular Life Sciences (2022)
-
Immunohistochemical expression and neurochemical phenotypes of huntingtin-associated protein 1 in the myenteric plexus of mouse gastrointestinal tract
Cell and Tissue Research (2021)
-
HAP1 Modulates Epileptic Seizures by Regulating GABAAR Function in Patients with Temporal Lobe Epilepsy and in the PTZ-Induced Epileptic Model
Neurochemical Research (2020)
-
Alzheimer’s Disease and Diabetes: Insulin Signaling as the Bridge Linking Two Pathologies
Molecular Neurobiology (2020)