Abstract
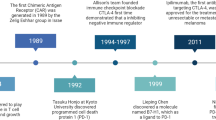
The US Food and Drug Administration (FDA) recently approved two novel immunotherapy agents, sipuleucel-T and ipilimumab, which showed a survival benefit for patients with metastatic prostate cancer and melanoma, respectively. The mechanisms by which these agents provideclinical benefit are not completely understood. However, knowledge of these mechanisms will be crucial for probing human immune responses and tumour biology in order to understand what distinguishes responders from non-responders. The following next steps are necessary: first, the development of immune-monitoring strategies for the identification of relevant biomarkers; second, the establishment of guidelines for the assessment of clinical end points; and third, the evaluation of combination therapy strategies to improve clinical benefit.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Burnet, F. M. Immunological aspects of malignant disease. Lancet 1, 1171–1174 (1967).
Burnet, F. M. The concept of immunological surveillance. Prog. Exp. Tumor Res. 13, 1–27 (1970).
Thomas, L. On immunosurveillance in human cancer. Yale J. Biol. Med. 55, 329–333 (1982).
Kaplan, D. H. et al. Demonstration of an interferon-γ-dependent tumor surveillance system in immunocompetent mice. Proc. Natl Acad. Sci. USA 95, 7556–7561 (1998).
Shankaran, V. et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111 (2001).
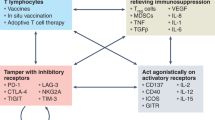
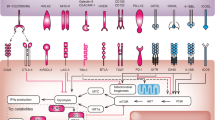
Lasaro, M. O. & Ertl, H. C. Targeting inhibitory pathways in cancer immunotherapy. Curr. Opin. Immunol. 22, 385–390 (2010).
Schreibelt, G. et al. Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol. Immunother. 59, 1573–1582 (2010).
Speiser, D. E. & Romero, P. Molecularly defined vaccines for cancer immunotherapy, and protective T cell immunity. Semin. Immunol. 22, 144–154 (2010).
Rosenberg, S. A. & Dudley, M. E. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr. Opin. Immunol. 21, 233–240 (2009).
Dudley, M. E. et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298, 850–854 (2002).
Fyfe, G. et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 13, 688–696 (1995).
Robbins, P. F. et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 29, 917–924 (2011).
Kershaw, M. H. et al. A phase I study of adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 12, 6106–6115 (2006).
Morgan, R. A. et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314, 126–129 (2006).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011).
Schwartzentruber, D. et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N. Engl. J. Med. 364, 2119–2127 (2011).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Kantoff, P. W. et al. Overall survival analysis of a phase II randomized controlled trial of a proxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 28, 1099–1105 (2010).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA4 blockade. Science 271, 1734–1736 (1996).
Van Elsas, A., Hurwitz, A. A. & Allison, J. P. Combination immunotherapy of B16 melanoma using anti-cytotoxic lymphocyte-associated antigen 4 (CTLA4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 190, 355–366 (1999).
Quezada, S. A. et al. CTLA4-blockade and GMCSF combination immunotherapy alters the intra-tumor balance of effector and regulatory T cells. J. Clin. Invest. 116, 1935–1945 (2006).
Kirkwood, J. M. et al. Phase II trial of tremelimumab (CP675206) in patients with advanced refractory or relapsed melanoma. Clin. Cancer Res. 16, 1042–1048 (2010).
Camacho, L. H. et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J. Clin. Oncol. 27, 1075–1081 (2009).
Ribas, A. Clinical development of the anti-CTLA4 antibody tremelimumab. Semin. Oncol. 37, 450–454 (2010).
Marshall, M. A., Ribas, A. & Huang, B. Evaluation of baseline serum C-reactive protein (CRP) and benefit from tremelimumab compared to chemotherapy in first-line melanoma. J. Clin. Oncol. 28 (Suppl.), Abstract 2609 (2010).
Liakou, C. I. et al. CTLA4 blockade increases IFNγ-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc. Natl Acad. Sci. USA 105, 14987–14992 (2008).
Chen, H. et al. Anti-CTLA4 therapy results in higher CD4+ICOShi T cell frequency and IFN-γ levels in both nonmalignant and malignant prostate tissues. Proc. Natl Acad. Sci. USA 106, 2729–2734 (2009).
Carthon, B. C. et al. Preoperative CTLA4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin. Cancer Res. 16, 2861–2871 (2010).
Vonderheide, R. H. et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin. Cancer Res. 16, 3485–3494 (2010).
Yuan, J. et al. CTLA4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc. Natl Acad. Sci. USA 105, 20410–20415 (2008).
Peggs, K. S., Quezada, S. A., Sharma, P. & Allison, J. P. Cancer immunotherapy. In Cancer Medicine 8th edn 175–189 (People's Medical Publishing House, Shelton, Connecticut, 2010).
Wolchok, J. D. et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 11, 155–164 (2010).
Salgaller, M., Marincola, F., Cormier, J. & Rosenberg, S. Immunization against epitopes in the human melanoma antigen gp100 following patient immunization with synthetic peptides. Cancer Res. 56, 4749–4757 (1996).
Korn, E. L. et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J. Clin. Oncol. 26, 527–534 (2008).
Robert, C. et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526 (2011).
Sznol, M. et al. Safety and antitumor activity of biweekly MDX1106 (Anti-PD1, BMS936558/ONO4538) in patients with advanced refractory malignancies. J. Clin. Oncol. 28 (Suppl.), Abstract 2506 (2010).
Saenger, Y. M. & Wolchok, J. D. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 8, 1 (2008).
O'Day, S. et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann. Oncol. 21, 1712–1717 (2010).
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
Kane, R. C. et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin. Cancer Res. 12, 7271–7278 (2006).
Spira, D. et al. Comparison of different tumor response criteria in patients with hepatocellular carcinoma after systemic therapy with the multikinase inhibitor sorafenib. Acad. Radiol. 18, 89–96 (2010).
Suzuki, C. et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics 28, 329–344 (2008).
Gajewski, T. F., Louahed, J. & Brichard, V. G. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 16, 399–403 (2010).
Haining, W. N. & Wherry, E. J. Integrating genomic signatures for immunologic discovery. Immunity 32, 152–161 (2010).
Kirkwood, J. M. et al. Immunogenicity and antitumor effects of vaccination with peptide vaccine +/− granulocyte-monocyte colony-stimulating factor and/or IFNα2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin. Cancer Res. 15, 1443–1451 (2009).
Slingluff, C. L. et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin. Cancer Res. 13, 6386–6395 (2007).
Britten, C. M., Janetzki, S., van der Burg, S. H., Gouttefangeas, C. & Hoos, A. Toward the harmonization of immune monitoring in clinical trials: quo vadis? Cancer Immunol. Immunother. 57, 285–288 (2008).
Janetzki, S. et al. “MIATA”-minimal information about T cell assays. Immunity 31, 527–528 (2009).
Janetzki, S., Cox, J. H., Oden, N. & Ferrari, G. Standardization and validation issues of the ELISPOT assay. Methods Mol. Biol. 302, 51–86 (2005).
Janetzki, S. et al. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI). Cancer Immunol. Immunother. 57, 303–315 (2008).
Moodie, Z. et al. Response definition criteria for ELISPOT assays revisited. Cancer Immunol. Immunother. 59, 1489–1501 (2010).
Koup, R. A, Graham, B. S. & Douek, D. C. The quest for a T cell-based immune correlate of protection against HIV: a story of trials and errors. Nature Rev. Immunol. 11, 65–70 (2011).
Dunn, G. P., Sheehan, K. C., Old, L. J. & Schreiber, R. D. IFN unresponsiveness in LNCaP cells due to the lack of JAK1 gene expression. Cancer Res. 65, 3447–3453 (2005).
Dancey, J. E. et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin. Cancer Res. 16, 1745–1755 (2010).
Sjöblom, T. et al. The consensus coding sequences of human breast and colorectal cancers. Science 314, 268–274 (2006).
Segal, N. H. Epitope landscape in breast and colorectal cancer. Cancer Res. 68, 889–892 (2008).
Peggs, K. S., Segal, N. H. & Allison, J. P. Targeting immunosupportive cancer therapies: accentuate the positive, eliminate the negative. Cancer Cell 12, 192–199 (2007).
Hodi, F. S. et al. A phase I trial of ipilimumab plus bevacizumab in patients with unresectable stage III or stage IV melanoma. J. Clin. Oncol. 29 (Suppl.), Abstract 8511 (2011).
Ngiow, S. et al. Anti-TIM3 antibody promotes T cell IFNγ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 71, 3540–3551 (2011).
Wang, L. et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 208, 577–592 (2011).
Acknowledgements
The authors' research work was supported by the Howard Hughes Medical Institute (for J.P.A.), the Ludwig Center for Cancer Immunotherapy (for P.S., J.D.W. and J.P.A.), a Prostate Cancer Foundation Challenge Award in Immunology (to P.S. and J.P.A.). P.S. also acknowledges support from an M. D. Anderson Cancer Center Physician Scientist Award, a Doris Duke Charitable Foundation Clinical Scientist Development Award, a Clinical Investigator Award from the Cancer Research Institute, a Melanoma Research Alliance Young Investigator Award, an American Cancer Society Mentored Research Scholar Grant and a US Department of Defense Prostate Cancer Idea Development Award.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
P.S., J.D.W. and J.P.A. have all served as paid consultants for Bristol-Myers Squibb (BMS). P.S. has also served as a paid consultant for Dendreon, Inc. J.P.A. is the inventor of anti-CTLA4 and has family members who own stock in BMS. K.W. is currently employed by Genentech.
Related links
DATABASES
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Sharma, P., Wagner, K., Wolchok, J. et al. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer 11, 805–812 (2011). https://doi.org/10.1038/nrc3153
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3153
This article is cited by
-
An engineered influenza virus to deliver antigens for lung cancer vaccination
Nature Biotechnology (2024)
-
The role of stromal cells in epithelial–mesenchymal plasticity and its therapeutic potential
Discover Oncology (2024)
-
CCL19/CCR7 drives regulatory T cell migration and indicates poor prognosis in gastric cancer
BMC Cancer (2023)
-
Comprehensive characterization of B7 family members in NSCLC and identification of its regulatory network
Scientific Reports (2023)
-
Tumor microenvironment-related gene selenium-binding protein 1 (SELENBP1) is associated with immunotherapy efficacy and survival in colorectal cancer
BMC Gastroenterology (2022)