Key Points
-
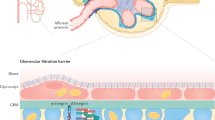
The luminal surface of the glomerular endothelium is covered with a hydrogel called the glycocalyx, which is comprised of glycosoaminoglycans, glycoproteins and associated serum proteins
-
The glycocalyx acts as a barrier against protein filtration across the endothelium
-
Loss of glycocalyx function might underlie the association of albuminuria with progression of renal and cardiovascular disease
-
Albuminuria as a consequence of glycocalyx dysfunction is likely to be a causal factor in the progression of renal disease
-
Understanding the mechanisms of disease that result from glycocalyx dysfunction could help to develop clinical trials in which albuminuria is an end point
Abstract
Albuminuria is commonly used as a marker of kidney disease progression, but some evidence suggests that albuminuria also contributes to disease progression by inducing renal injury in specific disease conditions. Studies have confirmed that in patients with cardiovascular risk factors, such as diabetes and hypertension, endothelial damage drives progression of kidney disease and cardiovascular disease. A key mechanism that contributes to this process is the loss of the glycocalyx—a polysaccharide gel that lines the luminal endothelial surface and that normally acts as a barrier against albumin filtration. Degradation of the glycocalyx in response to endothelial activation can lead to albuminuria and subsequent renal and vascular inflammation, thus providing a pathophysiological framework for the clinical association of albuminuria with renal and cardiovascular disease progression. In this Review, we examine the likely mechanisms by which glycocalyx dysfunction contributes to kidney injury and explains the link between cardiovascular disease and albuminuria. Evidence suggests that glycocalyx dysfunction is reversible, suggesting that these mechanisms could be considered as therapeutic targets to prevent the progression of renal and cardiovascular disease. This possibility enables the use of existing drugs in new ways, provides an opportunity to develop novel therapies, and indicates that albuminuria should be reconsidered as an end point in clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Heerspink, H. J., Holtkamp, F. A., de Zeeuw, D. & Ravid, M. Monitoring kidney function and albuminuria in patients with diabetes. Diabetes Care 34 (Suppl. 2), S325–S329 (2011).
Patrakka, J. & Tryggvason, K. New insights into the role of podocytes in proteinuria. Nat. Rev. Nephrol. 5, 463–468 (2009).
Comper, W. D. The limited role of the glomerular endothelial cell glycocalyx as a barrier to transglomerular albumin transport. Connect. Tissue Res. 55, 2–7 (2014).
Mancia, G. et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. Blood Press. 18, 308–347 (2009).
Parving, H. H., Persson, F. & Rossing, P. Microalbuminuria: a parameter that has changed diabetes care. Diabetes Res. Clin. Pract. 107, 1–8 (2015).
Dane, M. J. et al. Glomerular endothelial surface layer acts as a barrier against albumin filtration. Am. J. Pathol. 182, 1532–1540 (2013).
Satchell, S. C. The glomerular endothelium emerges as a key player in diabetic nephropathy. Kidney Int. 82, 949–951 (2012).
van den Berg, B. M., Spaan, J. A. & Vink, H. Impaired glycocalyx barrier properties contribute to enhanced intimal low-density lipoprotein accumulation at the carotid artery bifurcation in mice. Pflugers Arch. 457, 1199–1206 (2009).
Harvey, S. J. et al. Role of distinct type IV collagen networks in glomerular development and function. Kidney Int. 54, 1857–1866 (1998).
Satchell, S. The role of the glomerular endothelium in albumin handling. Nat. Rev. Nephrol. 12, 717–725 (2013).
Esko, J. D. & Selleck, S. B. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 71, 435–471 (2002).
Wang, L., Fuster, M., Sriramarao, P. & Esko, J. D. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat. Immunol. 6, 902–910 (2005).
Garsen, M., Rops, A. L., Rabelink, T. J., Berden, J. H. & van der Vlag, J. The role of heparanase and the endothelial glycocalyx in the development of proteinuria. Nephrol. Dial. Transplant. 29, 49–55 (2014).
Kiessling, L. L. & Grim, J. C. Glycopolymer probes of signal transduction. Chem. Soc. Rev. 42, 4476–4491 (2013).
Xu, D. & Esko, J. D. Demystifying heparan sulfate-protein interactions. Annu. Rev. Biochem. 83, 129–157 (2014).
Hoogewerf, A J. et al. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry 36, 13570–13578 (1997).
Lortat-Jacob, H. The molecular basis and functional implications of chemokine interactions with heparan sulphate. Curr. Opin. Struct. Biol. 19, 543–548 (2009).
Kosto, K. B. & Deen, W. M. Hindered convection of macromolecules in hydrogels. Biophys. J. 88, 277–286 (2005).
Adamson, R. H. et al. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J. Physiol. 557, 889–907 (2004).
Ryan, G. B. & Karnovsky, M. J. Distribution of endogenous albumin in the rat glomerulus: role of hemodynamic factors in glomerular barrier function. Kidney Int. 9, 36–45 (1976).
Jeansson, M. & Haraldsson, B. Morphological and functional evidence for an important role of the endothelial cell glycocalyx in the glomerular barrier. Am. J. Physiol. Renal Physiol. 290, F111–F116 (2006).
Ruggiero, A. et al. Paradoxical glomerular filtration of carbon nanotubes. Proc. Natl Acad. Sci. USA 107, 12369–12374 (2010).
Fridén, V. et al. The glomerular endothelial cell coat is essential for glomerular filtration. Kidney Int. 79, 1322–1330 (2011).
Chang, R. L. et al. Permselectivity of the glomerular capillary wall to macromolecules. II. Experimental studies in rats using neutral dextran. Biophys. J. 15, 887–906 (1975).
Guimarães, M. A., Nikolovski, J., Pratt, L. M., Greive, K. & Comper, W. D. Anomalous fractional clearance of negatively charged Ficoll relative to uncharged Ficoll. Am. J. Physiol. Renal Physiol. 285, F1118–F1124 (2003).
Rabelink, T. J., de Boer, H. C. & van Zonneveld, A. J. Endothelial activation and circulating markers of endothelial activation in kidney disease. Nat. Rev. Nephrol. 6, 404–414 (2010).
Gil, N. et al. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes 61, 208–216 (2012).
Axelsson, J. et al. Inactivation of heparan sulfate 2-O-sulfotransferase accentuates neutrophil infiltration during acute inflammation in mice. Blood 120, 1742–1751 (2012).
Rops, A. L. et al. Modulation of heparan sulfate in the glomerular endothelial glycocalyx decreases leukocyte influx during experimental glomerulonephritis. Kidney Int. 86, 932–942 (2014).
Wijnhoven, T. J. et al. Heparanase induces a differential loss of heparan sulphate domains in overt diabetic nephropathy. Diabetologia 51, 372–382 (2008).
Chajara, A. et al. Circulating hyaluronan and hyaluronidase are increased in diabetic rats. Diabetologia 43, 387–388 (2000).
Ikegami-Kawai, M., Suzuki, A., Karita, I. & Takahashi, T. Increased hyaluronidase activity in the kidney of streptozotocin-induced diabetic rats. J. Biochem. 134, 875–880 (2003).
Fiebiger, E. et al. Invariant chain controls the activity of extracellular cathepsin L. J. Exp. Med. 196, 1263–1269 (2002).
Harada, H. & Takahashi, M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J. Biol. Chem. 282, 5597–5607 (2007).
van den Hoven, M. J. et al. Increased expression of heparanase in overt diabetic nephropathy. Kidney Int. 70, 2100–2108 (2006).
Parish, C. R. The role of heparan sulphate in inflammation. Nat. Rev. Immunol. 6, 633–643 (2006).
Kriz, W. & LeHir, M. Pathways to nephron loss starting from glomerular diseases—insights from animal models. Kidney Int. 67, 404–419 (2005).
Donath, M. Y. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat. Rev. Drug Discov. 13, 465–476 (2014).
Ninichuk, V. et al. Late onset of Ccl2 blockade with the Spiegelmer mNOX-E36-3′PEG prevents glomerulosclerosis and improves glomerular filtration rate in db/db mice. Am. J. Pathol. 172, 628–637 (2008).
Dane, M. J. et al. Association of kidney function with changes in the endothelial surface layer. Clin. J. Am. Soc. Nephrol. 9, 698–704 (2014).
Padberg, J. S. et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis 234, 335–343 (2014).
Nieuwdorp, M. et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 55, 1127–1132 (2006).
Nieuwdorp, M. et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 55, 480–486 (2006).
Vlahu, C. A. et al. Damage of the endothelial glycocalyx in dialysis patients. J. Am. Soc. Nephrol. 23, 1900–1908 (2012).
Lee, D. H. et al. Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS ONE 9, e96477 (2014).
Dane, M., van den Berg, B. & Rabelink, T. The endothelial glycocalyx: scratching the surface for cardiovascular disease in kidney failure. Atherosclerosis 235, 56–57 (2014).
Zoja, C., Benigni, A. & Remuzzi, G. Cellular responses to protein overload: key event in renal disease progression. Curr. Opin. Nephrol. Hypertens. 13, 31–37 (2004).
Morigi, M. et al. In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: implication for permselective dysfunction of chronic nephropathies. Am. J. Pathol. 166, 1309–1320 (2005).
Alaiz, M., Beppu, M., Ohishi, K. & Kikugawa, K. Modification of delipidated apoprotein B of low density lipoprotein by lipid oxidation products in relation to macrophage scavenger receptor binding. Biol. Pharm. Bull. 17, 51–57 (1994).
Müller-Krebs, S. et al. Human RAGE antibody protects against AGE-mediated podocyte dysfunction. Nephrol. Dial. Transplant 27, 3129–3136 (2012).
Gutwein, P. et al. CXCL16 is expressed in podocytes and acts as a scavenger receptor for oxidized low-density lipoprotein. Am. J. Pathol. 174, 2061–2072 (2009).
Remuzzi, G., Ruggenenti, P. & Benigni, A. Understanding the nature of renal disease progression. Kidney Int. 51, 2–15 (1997).
Theilig, F. et al. Abrogation of protein uptake through megalin-deficient proximal tubules does not safeguard against tubulointerstitial injury. J. Am. Soc. Nephrol. 18, 1824–1834 (2007).
Daehn, I. et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J. Clin. Invest. 124, 1608–1621 (2014).
Sun, Y. B. et al. Glomerular endothelial cell injury and damage precedes that of podocytes in adriamycin-induced nephropathy. PLoS ONE 8, e55027 (2013).
Kanetsuna, Y. et al. Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am. J. Pathol. 170, 1473–1484 (2007).
Oltean, S. et al. Vascular endothelial growth factor-A165b is protective and restores endothelial glycocalyx in diabetic nephropathy. J. Am. Soc. Nephrol. 26, 1889–1904 (2015).
van den Berg, B. M. et al. Genetic deletion of endothelial hyaluronan synthase 2 results in glomerular injury and albuminuria [oral abstract TH-OR119] J. Am. Soc. Nephrol. 25 (Suppl.) 29A (2014).
van den Berg, B. M., Spaan, J. A., Rolf, T. M. & Vink, H. Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am. J. Physiol. Heart Circ. Physiol. 290, H915–H920 (2006).
Vink, H., Constantinescu, A. A. & Spaan, J. A. Oxidized lipoproteins degrade the endothelial surface layer: implications for platelet-endothelial cell adhesion. Circulation 101, 1500–1502 (2000).
Constantinescu, A., Spaan, J. A., Arkenbout, E. K., Vink, H. & Vanteeffelen, J. W. Degradation of the endothelial glycocalyx is associated with chylomicron leakage in mouse cremaster muscle microcirculation. Thromb. Haemost. 105, 790–801 (2011).
Deckert, T., Feldt-Rasmussen, B., Borch-Johnsen, K., Jensen, T. & Kofoed-Enevoldsen, A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 32, 219–226 (1989).
Bakker, S. J., Gansevoort, R. T., Stuveling, E. M., Gans, R. O. & de Zeeuw, D. Microalbuminuria and C-reactive protein: similar messengers of cardiovascular risk? Curr. Hypertens. Rep. 7, 379–384 (2005).
Ryan, U. S. & Ryan, J. W. The ultrastructural basis of endothelial cell surface functions. Biorheology 21, 155–170 (1984).
Yoneda, H., Ueta, K., Nagasaki, M. & Arakawa, K. Involvement of heparan sulfate in the renoprotective effects of imidapril, an angiotensin-converting enzyme inhibitor, in diabetic db/db mice. J. Recept. Signal Transduct. Res. 34, 21–25 (2014).
Ruilope, L. M. Angiotensin receptor blockers: RAAS blockade and renoprotection. Curr. Med. Res. Opin. 24, 1285–1293 (2008).
van den Hoven, M. J. et al. Regulation of glomerular heparanase expression by aldosterone, angiotensin II and reactive oxygen species. Nephrol. Dial. Transplant. 24, 2637–2645 (2009).
van der Pijl, J. W. et al. Danaparoid sodium lowers proteinuria in diabetic nephropathy. J. Am. Soc. Nephrol. 8, 456–462 (1997).
Myrup, B. et al. Effect of low-dose heparin on urinary albumin excretion in insulin-dependent diabetes mellitus. Lancet 345, 421–422 (1995).
Tamsma J. T., van der Woude, F. J. & Lemkes, H. H. Effect of sulphated glycosaminoglycans on albuminuria in patients with overt diabetic (type 1) nephropathy. Nephrol. Dial. Transplant. 11, 182–185 (1996).
Naggi, A. et al. Modulation of the heparanase-inhibiting activity of heparin through selective desulfation, graded N-acetylation, and glycol splitting. J. Biol. Chem. 280, 12103–12113 (2005).
Rao, N. V. et al. Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin-induced thrombocytopenia, and inhibits interaction of RAGE with its ligands. Am. J. Physiol. Cell Physiol. 299, C97–C110 (2010).
Poplawska, A., Szelachowska, M., Topolska, J., Wysocka-Solowie, B. & Kinalska, I. Effect of glycosaminoglycans on urinary albumin excretion in insulin-dependent diabetic patients with micro- or macroalbuminuria. Diabetes Res. Clin. Pract. 38, 109–114 (1997).
Broekhuizen, L. N. et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 53, 2646–2655 (2010).
Lewis, E. J. et al. Sulodexide for kidney protection in type 2 diabetes patients with microalbuminuria: a randomized controlled trial. Am. J. Kidney Dis. 58, 729–736 (2011).
Packham, D. K. et al. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 23, 123–130 (2012).
Gambaro, G. et al. Oral sulodexide reduces albuminuria in microalbuminuric and macroalbuminuric type 1 and type 2 diabetic patients: the Di.N.A.S. randomized trial. J. Am. Soc. Nephrol. 13, 1615–1625 (2002).
Bang, K. et al. Anti-proteinuric effect of sulodexide in immunoglobulin A nephropathy. Yonsei Med. J. 52, 588–594 (2011).
Schulman, G. et al. Randomized placebo-controlled EPPIC trials of AST-120 in CKD. J. Am. Soc. Nephrol. 26, 1732–1746 (2015).
Gambaro, G. Discounting the efficacy of sulodexide in diabetic nephropathy is premature. Am. J. Kidney Dis. 60, 169–170 (2012).
Vlodavsky, I., Ilan, N., Naggi, A. & Casu, B. Heparanase: structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Curr. Pharm. Des. 13, 2057–2073 (2007).
VanTeeffelen, J. W., Brands, J., Jansen, C., Spaan, J. A. & Vink, H. Heparin impairs glycocalyx barrier properties and attenuates shear dependent vasodilation in mice. Hypertension 50, 261–267 (2007).
Weinbaum, S., Zhang, X., Han, Y., Vink, H. & Cowin, S. C. Mechanotransduction and flow across the endothelial glycocalyx. Proc. Natl Acad. Sci. USA 100, 7988–7995 (2003).
Maguire, J. J. & Davenport, A. P. Endothelin receptors and their antagonists. Semin. Nephrol. 35, 125–136 (2015).
Verhaar, M. C. et al. Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation 97, 752–756 (1998).
Boels, M. G. et al. Reduction in albuminuria in diabetic nephropathy by atrasentan is associated with restoration of the glomerular endothelial glycocalyx [oral abstract SA-OR037]. J. Am. Soc. Nephrol. 25 (Suppl.), 89A (2014).
de Zeeuw, D. et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 25, 1083–1093 (2014).
U.K. Prospective Diabetes Study Group. Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37). Diabetes Care 22, 1125–1136 (1999).
Palmer, S. C. et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 385, 2047–2056 (2015).
Imai, E. et al. Reduction and residual proteinuria are therapeutic targets in type 2 diabetes with overt nephropathy: a post hoc analysis (ORIENT-proteinuria). Nephrol. Dial. Transplant. 28, 2526–2534 (2013).
Baigent, C. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377, 2181–2192 (2011).
Pfeffer, M. A. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N. Engl. J. Med. 361, 2019–2032 (2009).
Fried, L. F. et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 369, 1892–1903 (2013).
Mann, J. F. et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 372, 547–553 (2008).
Schmieder, R. E. et al. Mortality and morbidity in relation to changes in albuminuria, glucose status and systolic blood pressure: an analysis of the ONTARGET and TRANSCEND studies. Diabetologia 57, 2019–2029 (2014).
Usui, T. H., Hoekman, J., de Zeeuw, D. & Lambers Heerspink, H. J. Stroke outcomes and renal function in diabetes: a meta-analysis of randomized controlled trials [Poster FR-PO751]. J. Am. Soc. Nephrol. 25 (Suppl.), 541A (2014).
Thompson, A. Proteinuria as a surrogate end point—more data are needed. Nat. Rev. Nephrol. 8, 306–309 (2012).
US National Library of Medicine. ClinicalTrials.gov [online] (2015).
Vreys, V. et al. Cellular uptake of mammalian heparanase precursor involves low density lipoprotein receptor-related proteins, mannose 6-phosphate receptors, and heparan sulfate proteoglycans. J. Biol. Chem. 280, 33141–33148 (2005).
Roucourt, B., Meeussen, S., Bao, J., Zimmermann, P. & David, G. Heparanase activates the syndecan–syntenin–ALIX exosome pathway. Cell Res. 25, 412–428 (2015).
Goodall, K. J., Poon, I. K., Phipps, S. & Hulett, M. D. Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PLoS ONE 9, e109596 (2014).
Félétou, M., Köhler, R. & Vanhoutte, P. M. Endothelium-derived vasoactive factors and hypertension: possible roles in pathogenesis and as treatment targets. Curr. Hypertens. Rep. 12, 267–275 (2010).
Stamler, J. S., Lamas, S. & Fang, F. C. Nitrosylation. The prototypic redox-based signaling mechanism. Cell 106, 675–683 (2001).
Marshall, H. E. & Stamler, J. S. Inhibition of NF-κB by S-nitrosylation. Biochemistry 40, 1688–1693 (2001).
Matsushita, K. et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 115, 139–150 (2003).
Rabelink, T. J. & Luscher, T. F. Endothelial nitric oxide synthase: host defense enzyme of the endothelium? Arterioscler. Thromb. Vasc. Biol. 26, 267–271 (2006).
Hansson, G. K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352, 1685–1695 (2005).
Acknowledgements
T.J.R. was supported by the Glycoren consortium grant of the Dutch Kidney Foundation (CP09.03).
Author information
Authors and Affiliations
Contributions
T.J.R. researched data for the article. Both authors made substantial contributions to discussion of content, wrote the article, and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Rabelink, T., de Zeeuw, D. The glycocalyx—linking albuminuria with renal and cardiovascular disease. Nat Rev Nephrol 11, 667–676 (2015). https://doi.org/10.1038/nrneph.2015.162
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2015.162
This article is cited by
-
Optimal threshold of urinary albumin-to-creatinine ratio (UACR) for predicting long-term cardiovascular and noncardiovascular mortality
International Urology and Nephrology (2023)
-
Endothelial dysfunction as a factor leading to arterial hypertension
Pediatric Nephrology (2023)
-
Albuminuria but not low eGFR is closely associated with atherosclerosis in patients with type 2 diabetes: an observational study
Diabetology & Metabolic Syndrome (2022)
-
Assessment of fluid unresponsiveness guided by lung ultrasound in abdominal surgery: a prospective cohort study
Scientific Reports (2022)
-
Immunomodulation by endothelial cells — partnering up with the immune system?
Nature Reviews Immunology (2022)