Key Points
-
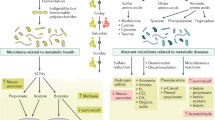
Dietary modifications (in particular high-fat feeding) can induce changes in the microbiota that might impact on host-associated metabolic disorders, such as insulin resistance
-
Germ-free mice exhibit reduced adiposity and fat pads compared to conventionally housed mice, thus implicating the gut microbiota in energy storage
-
An obese phenotype is transmissible via faecal transfer from obese mice or humans to germ-free mice
-
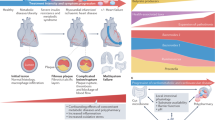
Metabolites that are derived from the gut microbiota, such as trimethylamine N-oxide (TMAO), are associated with poor cardiovascular outcomes in patients with cardiovascular disease
-
TMAO is increased in patients with chronic kidney disease (CKD) before haemodialysis and decreases after kidney transplantation
-
TMAO is associated with poor cardiovascular outcomes in patients with CKD
Abstract
Cardiometabolic diseases (CMDs) have been associated with changes in the composition of the gut microbiota, with links between the host environment and microbiota identified in preclinical models. High-throughput sequencing technology has facilitated in-depth studies of the gut microbiota, bacterial-derived metabolites, and their association with CMDs. Such strategies have shown that patients with CMDs frequently exhibit enrichment or depletion of certain bacterial groups in their resident microbiota compared to healthy individuals. Furthermore, the ability to transfer resident gut microbiota from mice or humans into germ-free mouse models, or between human patients, has enabled researchers to characterize the causative role of the gut microbiota in CMDs. These approaches have helped identify that dietary intake of choline, which is metabolized by the gut microbiota, is associated with cardiovascular outcomes in mice and humans. Trimethylamine N-oxide (TMAO) — a metabolite derived from the gut microbiota — is also associated with poor cardiovascular outcomes in patients with cardiovascular disease and is elevated in patients with chronic kidney disease (CKD). TMAO might represent a biomarker that links the environment and microbiota with CKD. This Review summarizes data suggesting a link between the gut microbiota and derived metabolites with food intake patterns, metabolic alterations, and chronic CMDs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ogden, C. L., Carroll, M. D. & Flegal, K. M. Prevalence of obesity in the United States. JAMA 312, 189–190 (2014).
Kopelman, P. G. Obesity as a medical problem. Nature 404, 635–643 (2000).
Arena, R. et al. Healthy lifestyle interventions to combat noncommunicable disease — a novel nonhierarchical connectivity model for key stakeholders: a policy statement from the American Heart Association, European Society of Cardiology, European Association for Cardiovascular Prevention and Rehabilitation, and American College of Preventive Medicine. Eur. Heart J. 36, 2097–2109 (2015).
Turnbaugh, P. J. et al. The human microbiome project. Nature 449, 804–810 (2007).
Clemente, J. C., Ursell, L. K., Parfrey, L. W. & Knight, R. The impact of the gut microbiota on human health: an integrative view. Cell 148, 1258–1270 (2012).
Aron-Wisnewsky, J., Doré, J. & Clement, K. The importance of the gut microbiota after bariatric surgery. Nat. Rev. Gastroenterol. Hepatol. 9, 590–598 (2012).
Fouhy, F., Ross, R. P., Fitzgerald, G. F., Stanton, C. & Cotter, P. D. Composition of the early intestinal microbiota: knowledge, knowledge gaps and the use of high-throughput sequencing to address these gaps. Gut Microbes 3, 203–220 (2012).
Shendure, J. & Ji, H. Next-generation DNA sequencing. Nat. Biotechnol. 26, 1135–1145 (2008).
Li, J. et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 32, 834–841 (2014).
Parkhill, J. What has high-throughput sequencing ever done for us? Nat. Rev. Microbiol. 11, 664–665 (2013).
Thomas, L. V. & Ockhuizen, T. New insights into the impact of the intestinal microbiota on health and disease: a symposium report. Br. J. Nutr. 107, S1–S13 (2012).
Ji, B. & Nielsen, J. From next-generation sequencing to systematic modeling of the gut microbiome. Front. Genet. 6, 219 (2015).
Santiago, A. et al. Processing faecal samples: a step forward for standards in microbial community analysis. BMC Microbiol. 14, 112 (2014).
Cardona, S. et al. Storage conditions of intestinal microbiota matter in metagenomic analysis. BMC Microbiol. 12, 158 (2012).
Bromberg, J. S., Fricke, W. F., Brinkman, C. C., Simon, T. & Mongodin, E. F. Microbiota — implications for immunity and transplantation. Nat. Rev. Nephrol. 11, 342–353 (2015).
Bäckhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl Acad. Sci. USA 101, 15718–15723 (2004).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Turnbaugh, P. J., Bäckhed, F., Fulton, L. & Gordon, J. I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3, 213–223 (2008).
Ridaura, V. K. et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214 (2013).
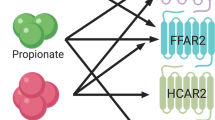
Chambers, E. S. et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut http://dx.doi.org/10.1136/gutjnl-2014-307913 (2014).
Psichas, A. et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 39, 424–429 (2015).
Sonnenburg, J. L. et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307, 1955–1959 (2005).
Gill, S. R. et al. Metagenomic analysis of the human distal gut microbiome. Science 312, 1355–1359 (2006).
Topping, D. L. & Clifton, P. M. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 81, 1031–1064 (2001).
Aguirre, M., Jonkers, D. M., Troost, F. J. & Roeselers, G., Venema K. In vitro characterization of the impact of different substrates on metabolite production, energy extraction and composition of gut microbiota from lean and obese subjects. PLoS ONE 9, e113864 (2014).
Murphy, E. F. et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut 59, 1635–1642 (2010).
Everard, A. et al. Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat. Commun. 5, 5648 (2014).
Tlaskalová-Hogenová, H. et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell. Mol. Immunol. 8, 110–120 (2011).
Steinmeyer, S., Lee, K., Jayaraman, A. & Alaniz, R. C. Microbiota metabolite regulation of host immune homeostasis: a mechanistic missing link. Curr. Allergy Asthma Rep. 15, 24 (2015).
Reikvam, D. H. et al. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS ONE 6, e17996 (2011).
Nguyen, T. L., Vieira-Silva, S., Liston, A. & Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model Mech. 8, 1–16 (2015).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107, 14691–14696 (2010).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Cotillard, A. et al. Dietary intervention impact on gut microbial gene richness. Nature 500, 585–588 (2013).
Kong, L. C. et al. Dietary patterns differently associate with inflammation and gut microbiota in overweight and obese subjects. PLoS ONE 9, e109434 (2014).
Claesson, M. J. et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184 (2012).
Arumugam, M. et al. Enterotypes of the human gut microbiome. Nature 473, 174–180 (2011).
Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012).
Karlsson, F. H. et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 3, 1245 (2012).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Wang, J. et al. Dietary history contributes to enterotype-like clustering and functional metagenomic content in the intestinal microbiome of wild mice. Proc. Natl Acad. Sci. USA 111, E2703–E2710 (2014).
Knights, D. et al. Rethinking 'enterotypes'. Cell Host Microbe 16, 433–437 (2014).
Jeffery, I. B., Claesson, M. J., O'Toole, P. W. & Shanahan, F. Categorization of the gut microbiota: enterotypes or gradients? Nat. Rev. Microbiol. 10, 591–592 (2012).
Koren, O. et al. A guide to enterotypes across the human body: meta-analysis of microbial community structures in human microbiome datasets. PLoS Comput. Biol. 9, e1002863 (2013).
Huse, S. M., Ye, Y., Zhou, Y. & Fodor, A. A. A core human microbiome as viewed through 16S rRNA sequence clusters. PLoS ONE 7, e34242 (2012).
Xu, Z. & Knight, R. Dietary effects on human gut microbiome diversity. Br. J. Nutr. 113, S1–S5 (2015).
Voreades, N., Kozil, A. & Weir, T. L. Diet and the development of the human intestinal microbiome. Front. Microbiol. 5, 494 (2014).
Ravussin, Y. et al. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity (Silver Spring) 20, 738–747 (2012).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Walker, A. W. et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 5, 220–230 (2011).
Duncan, S. H. et al. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl. Environ. Microbiol. 73, 1073–1078 (2007).
Duncan, S. H. et al. Human colonic microbiota associated with diet, obesity and weight loss. Int. J. Obes. 32, 1720–1724 (2008).
Shoaie, S. et al. Quantifying diet-induced metabolic changes of the human gut microbiome. Cell Metab. 22, 320–331 (2015).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Fernández-Real, J. M. et al. CD14 modulates inflammation-driven insulin resistance. Diabetes 60, 2179–2186 (2011).
Cani, P. D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008).
Carvalho, B. M. et al. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia 55, 2823–2834 (2012).
Gummesson, A. et al. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity (Silver Spring) 19, 2280–2282 (2011).
Bischoff, S. C. et al. Intestinal permeability — a new target for disease prevention and therapy. BMC Gastroenterol. 14, 189 (2014).
Amar, J. et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol. Med. 3, 559–572 (2011).
Cani, P. D. et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–1103 (2009).
Everard, A. et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 60, 2775–2786 (2011).
Everard, A. et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl Acad. Sci. USA 110, 9066–9071 (2013).
Derrien, M., Vaughan, E. E., Plugge, C. M. & de Vos, W. M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 54, 1469–1476 (2004).
Shin N.-R. et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63, 727–735 (2014).
Khan, M. T., Nieuwdorp, M. & Bäckhed, F. Microbial modulation of insulin sensitivity. Cell Metab. 20, 753–760 (2014).
Tilg, H. & Moschen, A. R. Microbiota and diabetes: an evolving relationship. Gut 63, 1513–1521 (2014).
Tremaroli, V. & Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 (2012).
Tilg, H. & Kaser, A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Invest. 121, 2126–2132 (2011).
Le Chatelier, E. et al. Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546 (2013).
Dao, M. C. et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut http://dx.doi.org/10.1136/gutjnl-2014-308778 (2015).
Zhang, H. et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl Acad. Sci. USA 106, 2365–2370 (2009).
Liou, A. P. et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 5, 178ra41 (2013).
Aron-Wisnewsky, J. & Clement, K. The effects of gastrointestinal surgery on gut microbiota: potential contribution to improved insulin sensitivity. Curr. Atheroscler. Rep. 16, 454 (2014).
Chambers, E. S. et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut http://dx.doi.org/10.1136/gutjnl-2014-307913 (2014).
Karaki S.-I. et al. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J. Mol. Histol. 39, 135–142 (2008).
Goodrich, J. K. et al. Human genetics shape the gut microbiome. Cell 159, 789–799 (2014).
Sokol, H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA 105, 16731–16736 (2008).
Quévrain, E. et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn's disease. Gut http://dx.doi.org/10.1136/gutjnl-2014-307649 (2015).
Karlsson, F. H. et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498, 99–103 (2013).
Zhang, X. et al. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE 8, e71108 (2013).
Furet, J.-P. et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 59, 3049–3057 (2010).
Sjöström, L. et al. Bariatric surgery and long-term cardiovascular events. JAMA 307, 56–65 (2012).
Sjöström, L. et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 311, 2297–2304 (2014).
Buse, J. B. et al. How do we define cure of diabetes? Diabetes Care 32, 2133–2135 (2009).
Tirosh, A. et al. Renal function following three distinct weight loss dietary strategies during 2 years of a randomized controlled trial. Diabetes Care 36, 2225–2232 (2013).
Chassaing, B., Ley, R. E. & Gewirtz, A. T. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology 147, 1363–1377.e17 (2014).
Kim, M. -S., Hwang, S. -S., Park, E. -J. & Bae, J.-W. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation. Environ. Microbiol. Rep. 5, 765–775 (2013).
Amar, J. et al. Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia 54, 3055–3061 (2011).
Surawicz, C. M. et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am. J. Gastroenterol. 108, 478–498 (2013).
Youngster, I. et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 312, 1772–1778 (2014).
van Nood, E. et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415 (2013).
Vrieze, A. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916.e7 (2012).
Maslowski, K. M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009).
Koren, O. et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl Acad. Sci. USA 108, S4592–S4598 (2011).
Spinler, S. A. et al. Frequency of attainment of low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol goals in cardiovascular clinical practice (from the National Cardiovascular Data Registry PINNACLE Registry). Am. J. Cardiol. 116, 547–553 (2015).
Tang, W. H. & Hazen, S. L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Invest. 124, 4204–4211 (2014).
Mendelsohn, A. R. & Larrick, J. W. Dietary modification of the microbiome affects risk for cardiovascular disease. Rejuvenation Res. 16, 241–244 (2013).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Lang, D. H. et al. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: selective catalysis by FMO3. Biochem. Pharmacol. 56, 1005–1012 (1998).
Stock J. Gut microbiota: an environmental risk factor for cardiovascular disease. Atherosclerosis 229, 440–442 (2013).
Bennett, B. J. et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 17, 49–60 (2013).
Trøseid, M. et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 277, 717–726 (2015).
Tang, W. H. et al. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J. Card. Fail. 21, 91–96 (2015).
Tang, W. H. et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J. Am. Coll. Cardiol. 64, 1908–1914 (2014).
Tang, W. H. et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 368, 1575–1584 (2013).
Wang, Z. et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 35, 904–910 (2014).
Gregory, J. C. et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J. Biol. Chem. 290, 5647–5660 (2015).
Daviglus, M. L. et al. Fish consumption and the 30-year risk of fatal myocardial infarction. N. Engl. J. Med. 336, 1046–1053 (1997).
Mozaffarian, D. & Wu, J. H. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 58, 2047–2067 (2011).
Koeth, R. A. et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585 (2013).
Du, Y., Wang, L. & Hong, B. High-density lipoprotein-based drug discovery for treatment of atherosclerosis. Expert Opin. Drug Discov. 28, 1–15 (2015).
Aron-Wisnewsky, J. et al. Effect of bariatric surgery-induced weight loss on SR-BI-, ABCG1-, and ABCA1-mediated cellular cholesterol efflux in obese women. J. Clin. Endocrinol. Metab. 96, 1151–1159 (2011).
Ussher, J. R., Lopaschuk, G. D. & Arduini, A. Gut microbiota metabolism of L-carnitine and cardiovascular risk. Atherosclerosis 231, 456–461 (2013).
Ringseis, R., Keller, J. & Eder, K. Role of carnitine in the regulation of glucose homeostasis and insulin sensitivity: evidence from in vivo and in vitro studies with carnitine supplementation and carnitine deficiency. Eur. J. Nutr. 51, 1–18 (2012).
Muoio, D. M. et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 15, 764–777 (2012).
Broderick, T. L., Quinney, H. A., Barker, C. C. & Lopaschuk, G. D. Beneficial effect of carnitine on mechanical recovery of rat hearts reperfused after a transient period of global ischemia is accompanied by a stimulation of glucose oxidation. Circulation 87, 972–981 (1993).
DiNicolantonio, J. J., Lavie, C. J., Fares, H., Menezes, A. R. & O'Keefe, J. H. L-carnitine in the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Mayo Clin. Proc. 88, 544–551 (2013).
Shang, R., Sun, Z. & Li, H. Effective dosing of L-carnitine in the secondary prevention of cardiovascular disease: a systematic review and meta-analysis. BMC Cardiovasc. Disord. 14, 88 (2014).
Lo, J. et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS Lond. Engl. 24, 243–253 (2010).
Srinivasa, S. et al. Plaque burden in HIV-infected patients is associated with serum intestinal microbiota-generated trimethylamine. AIDS Lond. Engl. 29, 443–452 (2015).
Stevens, P. E. & Levin, A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes clinical practice guideline. Ann. Intern. Med. 158, 825–830 (2012).
Akbari, A. et al. Canadian Society of Nephrology commentary on the KDIGO clinical practice guideline for CKD evaluation and management. Am. J. Kidney Dis. 65, 177–205 (2015).
Vaziri, N. D. et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 83, 308–315 (2013).
Wong, J. et al. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 39, 230–237 (2014).
Barros, A. F. et al. Is there interaction between gut microbial profile and cardiovascular risk in chronic kidney disease patients? Future Microbiol. 10, 517–526 (2015).
Stenvinkel, P., Lindholm, B., Heimbürger, M. & Heimbürger, O. Elevated serum levels of soluble adhesion molecules predict death in pre-dialysis patients: association with malnutrition, inflammation, and cardiovascular disease. Nephrol. Dial. Transplant. 15, 1624–1630 (2000).
Tripepi, G., Mallamaci, F. & Zoccali, C. Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: searching for the best risk marker by multivariate modeling. J. Am. Soc. Nephrol. 16, S83–S88 (2005).
Wang, F. et al. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrol. (Carlton) 17, 733–738 (2012).
Shi, K. et al. Gut bacterial translocation may aggravate microinflammation in hemodialysis patients. Dig. Dis. Sci. 59, 2109–2117 (2014).
Vaziri, N. D. Gut microbial translocation in the pathogenesis of systemic inflammation in patients with end-stage renal disease. Dig. Dis. Sci. 59, 2020–2022 (2014).
Savassi-Rocha, A. L. et al. Changes in intestinal permeability after Roux-en-Y gastric bypass. Obes. Surg. 24, 184–190 (2014).
Magnusson, M., Magnusson, K. E., Sundqvist, T. & Denneberg, T. Impaired intestinal barrier function measured by differently sized polyethylene glycols in patients with chronic renal failure. Gut 32, 754–759 (1991).
Magnusson, M., Magnusson, K. E., Sundqvist, T. & Denneberg, T. Increased intestinal permeability to differently sized polyethylene glycols in uremic rats: effects of low- and high-protein diets. Nephron 56, 306–311 (1990).
Fogelman, A. M. TMAO is both a biomarker and a renal toxin. Circ. Res. 116, 396–397 (2015).
Bain, M. A., Faull, R., Fornasini, G., Milne, R. W. & Evans, A. M. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol. Dial. Transplant. 21, 1300–1304 (2006).
Kaysen, G. A. et al. Associations of trimethylamine N-oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J. Ren. Nutr. 25, 351–356 (2015).
Stubbs, J. R. et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J. Am. Soc. Nephrol. http://dx.doi.org/10.1681/ASN.2014111063 (2015).
Bain, M. A., Faull, R., Milne, R. W. & Evans, A. M. Oral L -carnitine: metabolite formation and hemodialysis. Curr. Drug Metab. 7, 811–816 (2006).
Tang, W. H. et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 116, 448–455 (2015).
Onopiuk, A., Tokarzewicz, A. & Gorodkiewicz, E. Cystatin C: a kidney function biomarker. Adv. Clin. Chem. 68, 57–69 (2015).
Levey, A. S., Fan, L., Eckfeldt, J. H. & Inker, L. A. Cystatin C for glomerular filtration rate estimation: coming of age. Clin. Chem. 60, 916–919 (2014).
Scarpellini, E. et al. The human gut microbiota and virome: potential therapeutic implications. Dig. Liver Dis. http://dx.doi.org/10.1016/j.dld.2015.07.008 (2015).
Focà, A. et al. Gut inflammation and immunity: what isthe role of the human gut virome? Med. Inflamm. 2015, 326032 (2015).
Minot, S. et al. The human gut virome: inter-individual variation and dynamic response to diet. Genome Res. 21, 1616–1625 (2011).
Hopkins, M. J., Sharp. R. & Macfarlane, G. T. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 48, 198–205 (2001).
Hopkins, M. J. & Macfarlane, G. T. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J. Med. Microbiol. 51, 448–454 (2002).
Antonopoulos, D. A. et al. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect. Immun. 77, 2367–2375 (2009).
Macfarlane, S. Antibiotic treatments and microbes in the gut. Environ. Microbiol. 16, 919–924 (2014).
Ward, E. K. et al. The effect of PPI use on human gut microbiota and weight loss in patients undergoing laparoscopic Roux-en-Y gastric bypass. Obes. Surg. 24, 1567–1571 (2014).
Tsuda, A. et al. Influence of proton-pump inhibitors on the luminal microbiota in the gastrointestinal tract. Clin. Transl. Gastroenterol. 6, e89 (2015).
Allais, L. et al. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ. Microbiol. http://dx.doi.org/10.1111/1462-2920.12934 (2015).
Osto, M. et al. Roux-en-Y gastric bypass surgery in rats alters gut microbiota profile along the intestine. Physiol. Behav. 119, 92–96 (2013).
Tremaroli, V. et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 22, 228–238 (2015).
Thomas, V., Clark, J. & Doré, J. Fecal microbiota analysis: an overview of sample collection methods and sequencing strategies. Future Microbiol. 10, 1485–1504 (2015).
Kennedy, N. A. et al. The impact of different DNA extraction kits and laboratories upon the assessment of human gut microbiota composition by 16S rRNA gene sequencing. PLoS ONE 9, e88982 (2014).
Acknowledgements
The authors would like to thank the FP7 MetaCardis project (grant number HEALTH.F4_2012_305,312), the Coeur et Artère association (Fondacoeur), the AP–HP Microbaria project, and the National Agency of Research, which support their research into the gut microbiota and cardiometabolic health. The authors would also like to thank Brandon Kayser (Institute of Cardiometabolism and Nutrition, Pitié-Salpêtrière Hospital, Paris) for language editing the manuscript before submission.
Author information
Authors and Affiliations
Contributions
J.A.-W. wrote the article. J.A.-W. and K.C. researched the data for the article, provided substantial contribution to discussions of the content, and contributed equally to review and/or editing of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Aron-Wisnewsky, J., Clément, K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol 12, 169–181 (2016). https://doi.org/10.1038/nrneph.2015.191
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2015.191
This article is cited by
-
Antenatal gut microbiome profiles and effect on pregnancy outcome in HIV infected and HIV uninfected women in a resource limited setting
BMC Microbiology (2023)
-
Oral supplementation of nicotinamide riboside alters intestinal microbial composition in rats and mice, but not humans
npj Aging (2023)
-
Uygur type 2 diabetes patient fecal microbiota transplantation disrupts blood glucose and bile acid levels by changing the ability of the intestinal flora to metabolize bile acids in C57BL/6 mice
BMC Endocrine Disorders (2022)
-
Fecal microbiota transplantation ameliorates atherosclerosis in mice with C1q/TNF-related protein 9 genetic deficiency
Experimental & Molecular Medicine (2022)
-
Nitric oxide signalling in kidney regulation and cardiometabolic health
Nature Reviews Nephrology (2021)