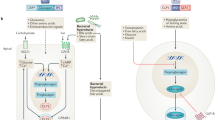
Key Points
-
The incretins glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are gut-derived hormones that potentiate insulin secretion and contribute to glucose metabolism through a wide range of physiological actions
-
Inhibitors of the incretin-inactivating enzyme dipeptidyl peptidase 4 (DPP-4) and DPP-4-resistant injectable GLP-1 receptor agonists have been developed for the treatment of hyperglycaemia in type 2 diabetes mellitus (T2DM)
-
GLP-1 and other gut-derived hormones might directly and/or indirectly regulate electrolyte and fluid homeostasis by influencing feeding and drinking behaviour as well as electrolyte transport in the kidneys and gastrointestinal tract
-
GLP-1 receptor (GLP-1R) agonists and DPP-4 inhibitors increase natriuresis in T2DM, possibly through overlapping and distinct mechanisms, and might slightly improve renal haemodynamics in the setting of diabetes-related glomerular hyperfiltration
-
Incretin-based therapies seem to directly influence renal physiology and have indirect metabolic and haemodynamic actions that might reduce renal risk in T2DM; considerable interest exists in identifying these glucose-independent renoprotective actions
-
Data from clinical trials suggest that GLP-1R agonists and, to a lesser extent, DPP-4 inhibitors marginally improve surrogate renal end points, plausibly beyond the effects of improved glycaemic control
Abstract
The gastrointestinal tract — the largest endocrine network in human physiology — orchestrates signals from the external environment to maintain neural and hormonal control of homeostasis. Advances in understanding entero-endocrine cell biology in health and disease have important translational relevance. The gut-derived incretin hormone glucagon-like peptide 1 (GLP-1) is secreted upon meal ingestion and controls glucose metabolism by modulating pancreatic islet cell function, food intake and gastrointestinal motility, amongst other effects. The observation that the insulinotropic actions of GLP-1 are reduced in type 2 diabetes mellitus (T2DM) led to the development of incretin-based therapies — GLP-1 receptor agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors — for the treatment of hyperglycaemia in these patients. Considerable interest exists in identifying effects of these drugs beyond glucose-lowering, possibly resulting in improved macrovascular and microvascular outcomes, including in diabetic kidney disease. As GLP-1 has been implicated as a mediator in the putative gut–renal axis (a rapid-acting feed-forward loop that regulates postprandial fluid and electrolyte homeostasis), direct actions on the kidney have been proposed. Here, we review the role of GLP-1 and the actions of associated therapies on glucose metabolism, the gut–renal axis, classical renal risk factors, and renal end points in randomized controlled trials of GLP-1 receptor agonists and DPP-4 inhibitors in patients with T2DM.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
International Diabetes Federation. IDF Diabetes Atlas 7th edn. Diabetes Atlas http://www.diabetesatlas.org/ (2015).
King, H., Aubert, R. E. & Herman, W. H. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care 21, 1414–1431 (1998).
Narayan, K. M., Boyle, J. P., Geiss, L. S., Saaddine, J. B. & Thompson, T. J. Impact of recent increase in incidence on future diabetes burden: U. S., 2005–2050. Diabetes Care 29, 2114–2116 (2006).
Muskiet, M. H. et al. Pleiotropic effects of type 2 diabetes management strategies on renal risk factors. Lancet Diabetes Endocrinol. 3, 367–381 (2015).
Nathan, D. M. Diabetes: advances in diagnosis and treatment. JAMA 314, 1052–1062 (2015).
Gregg, E. W. et al. Changes in diabetes-related complications in the United States, 1990–2010. N. Engl. J. Med. 370, 1514–1523 (2014).
Gregg, E. W., Sattar, N. & Ali, M. K. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 4, 537–547 (2016).
Thomas, M. C., Cooper, M. E. & Zimmet, P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 12, 73–81 (2016). Review of the epidemiology of T2DM and CKD in various regions worldwide, reporting that the decreasing prevalence of cardiovascular disease results in increased survival, which translates to an increased prevalence of renal complications.
Fernandez-Fernandez, B., Ortiz, A., Gomez-Guerrero, C. & Egido, J. Therapeutic approaches to diabetic nephropathy — beyond the RAS. Nat. Rev. Nephrol. 10, 325–346 (2014).
Fried, L. F. et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 369, 1892–1903 (2013).
European Medicine Agency. Combined use of medicines affecting the renin-angiotensin system (RAS) to be restricted — CHMP endorses PRAC recommendation. EMA http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2014/05/WC500167421.pdf (2014).
Inzucchi, S. E. et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38, 140–149 (2015).
Tahrani, A. A., Barnett, A. H. & Bailey, C. J. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 12, 566–592 (2016). Comprehensive review of currently available glucose-lowering therapies for the treatment of T2DM, focusing on mechanisms of action, pharmacokinetics, pharmacodynamics and safety profiles.
DeFronzo, R. A., Norton, L. & Abdul-Ghani, M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat. Rev. Nephrol. 13, 11–26 (2017).
Heerspink, H. J., Perkins, B. A., Fitchett, D. H., Husain, M. & Cherney, D. Z. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134, 752–772 (2016).
van Bommel, E. J. et al. SGLT2 inhibition in the diabetic kidney — from mechanisms to clinical outcome. Clin. J. Am. Soc. Nephrol. (2017).
Muskiet, M. H., Smits, M. M., Morsink, L. M. & Diamant, M. The gut-renal axis: do incretin-based agents confer renoprotection in diabetes? Nat. Rev. Nephrol. 10, 88–103 (2014). First review of the potential renoprotective effects and mechanisms of GLP-1R agonists and DPP-4 inhibitors in preclinical studies and in early clinical studies involving patients with diabetes.
Drucker, D. J. Evolving concepts and translational relevance of enteroendocrine cell biology. J. Clin. Endocrinol. Metab. 101, 778–786 (2016). Excellent review of the complexity of entero-endocrine cell biology, discussing gaps in current understanding and highlighting the translational relevance of entero-endocrine cells in the pathophysiology and therapy of metabolic and inflammatory disorders.
Furness, J. B., Rivera, L. R., Cho, H. J., Bravo, D. M. & Callaghan, B. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 10, 729–740 (2013).
Gribble, F. M. & Reimann, F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 78, 277–299 (2016).
LaBarre, J. Sur les possibilités d'un traitement du diabéte par l'incrétine. Bull. Acad. R. Med. Belg. 12, 620–634 (1932).
Nauck, M. A. & Meier, J. J. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 4, 525–536 (2016).
Nauck, M. A. et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J. Clin. Endocrinol. Metab. 63, 492–498 (1986).
Perley, M. J. & Kipnis, D. M. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J. Clin. Invest. 46, 1954–1962 (1967).
Buchan, A. M., Polak, J. M., Capella, C., Solcia, E. & Pearse, A. G. Electronimmunocytochemical evidence for the K cell localization of gastric inhibitory polypeptide (GIP) in man. Histochemistry 56, 37–44 (1978).
Eissele, R. et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur. J. Clin. Invest. 22, 283–291 (1992).
Cho, Y. M., Fujita, Y. & Kieffer, T. J. Glucagon-like peptide-1: glucose homeostasis and beyond. Annu. Rev. Physiol. 76, 535–559 (2014).
Smits, M. M. et al. Gastrointestinal actions of glucagon-like peptide-1-based therapies: glycaemic control beyond the pancreas. Diabetes Obes. Metab. 18, 224–235 (2016).
Nauck, M. A. et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J. Clin. Endocrinol. Metab. 87, 1239–1246 (2002).
Schirra, J. et al. Exendin(9–39)amide is an antagonist of glucagon-like peptide-1(7–36)amide in humans. J. Clin. Invest. 101, 1421–1430 (1998).
Salehi, M., Vahl, T. P. & D'Alessio, D. A. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J. Clin. Endocrinol. Metab. 93, 4909–4916 (2008).
de Heer, J., Rasmussen, C., Coy, D. H. & Holst, J. J. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia 51, 2263–2270 (2008).
Meier, J. J. et al. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia 46, 798–801 (2003).
Meier, J. J., Deacon, C. F., Schmidt, W. E., Holst, J. J. & Nauck, M. A. Suppression of glucagon secretion is lower after oral glucose administration than during intravenous glucose administration in human subjects. Diabetologia 50, 806–813 (2007).
van Bloemendaal, L., Ten Kulve, J. S., la Fleur, S. E., Ijzerman, R. G. & Diamant, M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J. Endocrinol. 221, T1–T16 (2014). Review describing the physiological role of GLP-1 in the central regulation of feeding behaviour, as well the effects of pharmacological stimulation of GLP-1 pathways leading to alterations in CNS activity, reduced food intake and weight loss in obesity and T2DM.
Ten Kulve, J. S. et al. Liraglutide reduces cns activation in response to visual food cues only after short-term treatment in patients with type 2 diabetes. Diabetes Care 39, 214–221 (2016).
van Bloemendaal, L. et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes 63, 4186–4196 (2014).
Smits, M. M. et al. GLP-1 based therapies: clinical implications for gastroenterologists. Gut 65, 702–711 (2016).
Nerup, N. et al. The effect of glucagon-like peptide-1 and glucagon-like peptide-2 on microcirculation: a systematic review. Microcirculation http://dx.doi.org/10.1111/micc.12367 (2017).
Nauck, M., Stockmann, F., Ebert, R. & Creutzfeldt, W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 29, 46–52 (1986). First study that showed that the incretin effect is markedly reduced in patients with T2DM.
Knop, F. K. et al. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 56, 1951–1959 (2007).
Bagger, J. I. et al. Impaired regulation of the incretin effect in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 96, 737–745 (2011).
Kahn, S. E., Cooper, M. E. & Del Prato, S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383, 1068–1083 (2014).
Calanna, S. et al. Secretion of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes: systematic review and meta-analysis of clinical studies. Diabetes Care 36, 3346–3352 (2013).
Calanna, S. et al. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: systematic review and meta-analyses of clinical studies. Diabetologia 56, 965–972 (2013).
Faerch, K. et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: the ADDITION-PRO study. Diabetes 64, 2513–2525 (2015).
Nauck, M. A. et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Invest. 91, 301–307 (1993).
Nauck, M. A. et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 36, 741–744 (1993).
Larsen, J., Hylleberg, B., Ng, K. & Damsbo, P. Glucagon-like peptide-1 infusion must be maintained for 24 h/day to obtain acceptable glycemia in type 2 diabetic patients who are poorly controlled on sulphonylurea treatment. Diabetes Care 24, 1416–1421 (2001).
Rachman, J., Barrow, B. A., Levy, J. C. & Turner, R. C. Near-normalisation of diurnal glucose concentrations by continuous administration of glucagon-like peptide-1 (GLP-1) in subjects with NIDDM. Diabetologia 40, 205–211 (1997).
Zander, M., Madsbad, S., Madsen, J. L. & Holst, J. J. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 359, 824–830 (2002). Key proof-of-principal study that shows that continuous subcutaneous infusion of GLP-1 improves glycaemic control in patients with T2DM.
Deacon, C. F., Nauck, M. A., Meier, J., Hucking, K. & Holst, J. J. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J. Clin. Endocrinol. Metab. 85, 3575–3581 (2000).
Mentlein, R., Gallwitz, B. & Schmidt, W. E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 214, 829–835 (1993).
Hupe-Sodmann, K. et al. Endoproteolysis of glucagon-like peptide (GLP)-1 (7–36) amide by ectopeptidases in RINm5F cells. Peptides 18, 625–632 (1997).
Hupe-Sodmann, K. et al. Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1(7–36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regul. Pept. 58, 149–156 (1995).
Mentlein, R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract. Res. Clin. Endocrinol. Metab. 23, 443–452 (2009).
Idorn, T. et al. Elimination and degradation of glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with end-stage renal disease. J. Clin. Endocrinol. Metab. 99, 2457–2466 (2014).
Mulvihill, E. E. & Drucker, D. J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 35, 992–1019 (2014).
Tella, S. H. & Rendell, M. S. Glucagon-like polypeptide agonists in type 2 diabetes mellitus: efficacy and tolerability, a balance. Ther. Adv. Endocrinol. Metab. 6, 109–134 (2015).
Meier, J. J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 8, 728–742 (2012). Early, in-depth review article that evaluates the similarities and differences between short-acting and long-acting GLP-1R agonists in terms of glucose-lowering efficacy and adverse effects, and discusses the possibilities for utilizing these agents for individualized treatment of T2DM.
Novo Nordisk. Novo nordisk files for regulatory approval of once-weekly semaglutide with the fda for the treatment of type 2 diabetes. Novo Nordisk http://press.novonordisk-us.com/2016-12-05-Novo-Nordisk-Files-for-Regulatory-Approval-of-Once-Weekly-Semaglutide-with-the-FDA-for-the-Treatment-of-Type-2-Diabetes (2016).
Davies, M., Chatterjee, S. & Khunti, K. The treatment of type 2 diabetes in the presence of renal impairment: what we should know about newer therapies. Clin. Pharmacol. 8, 61–81 (2016).
Scheen, A. J. Pharmacokinetics and clinical use of incretin-based therapies in patients with chronic kidney disease and type 2 diabetes. Clin. Pharmacokinet. 54, 1–21 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01394341 (2013).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016). First cardiovascular safety trial to demonstrate that a GLP-1R agonist reduces MACE and microvascular (including renal) events in patients with T2DM and high cardiovascular risk.
Mann, J. F. Liraglutide and renal outcomes in type 2 diabetes: results of the LEADER trial. Oral Abstract Session. Kidney Week, November 15–20 https://www.asn-online.org/education/kidneyweek/archives/ (2016).
Shyangdan, D. S. et al. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 5, CD006423 (2011).
Buse, J. B. et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 374, 39–47 (2009).
Drucker, D. J. et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 372, 1240–1250 (2008).
Meier, J. J. et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open-label trial. Diabetes Care 38, 1263–1273 (2015).
Kramer, C. K., Zinman, B., Choi, H., Connelly, P. W. & Retnakaran, R. The impact of chronic liraglutide therapy on glucagon secretion in type 2 diabetes: insight from the LIBRA trial. J. Clin. Endocrinol. Metab. 100, 3702–3709 (2015).
Smits, M. M. et al. Effect of 3 years of treatment with exenatide on postprandial glucagon levels. Diabetes Care 39, e42–e43 (2016).
Deacon, C. F. & Lebovitz, H. E. Comparative review of dipeptidyl peptidase-4 inhibitors and sulphonylureas. Diabetes Obes. Metab. 18, 333–347 (2016).
Deacon, C. F. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes. Metab. 13, 7–18 (2011).
Bergman, A. J. et al. Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care 30, 1862–1864 (2007).
Bailey, C. J. in International Textbook of Diabetes Mellitus 4th edn Ch 47 (eds DeFronzo, R.A., Ferrannini, E, Alberti, KG, Zimmet, P) (John Wiley & Sons Ltd., 2015).
Omar, B. & Ahren, B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes 63, 2196–2202 (2014).
Craddy, P., Palin, H. J. & Johnson, K. I. Comparative effectiveness of dipeptidylpeptidase-4 inhibitors in type 2 diabetes: a systematic review and mixed treatment comparison. Diabetes Ther. 5, 1–41 (2014).
Esposito, K. et al. A nomogram to estimate the HbA1c response to different DPP-4 inhibitors in type 2 diabetes: a systematic review and meta-analysis of 98 trials with 24 163 patients. BMJ Open 5, e005892 (2015).
Palmer, B. F. & Clegg, D. J. Physiology and pathophysiology of potassium homeostasis. Adv. Physiol. Educ. 40, 480–490 (2016).
Hoorn, E. J. & Zietse, R. Gut-kidney kaliuretic signaling: looking forward to feeding. Kidney Int. 88, 1230–1232 (2015).
Michell, A. R., Debnam, E. S. & Unwin, R. J. Regulation of renal function by the gastrointestinal tract: potential role of gut-derived peptides and hormones. Annu. Rev. Physiol. 70, 379–403 (2008).
Thomas, L. & Kumar, R. Control of renal solute excretion by enteric signals and mediators. J. Am. Soc. Nephrol. 19, 207–212 (2008).
Carey, R. M. Evidence for a splanchnic sodium input monitor regulating renal sodium excretion in man. Lack of dependence upon aldosterone. Circ. Res. 43, 19–23 (1978).
Preston, R. A. et al. Sodium challenge does not support an acute gastrointestinal-renal natriuretic signaling axis in humans. Kidney Int. 82, 1313–1320 (2012).
Singer, D. R. et al. Contrasting endocrine responses to acute oral compared with intravenous sodium loading in normal humans. Am. J. Physiol. 274, F111–F119 (1998). Clinical study that evaluated the endocrine mechanisms involved in accelerated natriuresis after oral compared with intravenous sodium loading in healthy individuals.
Yang, J., Jose, P. A. & Zeng, C. Gastrointestinal-renal axis: role in the regulation of blood pressure. J. Am. Heart Assoc. 6, e005536 (2017).
Jose, P. A., Felder, R. A., Yang, Z., Zeng, C. & Eisner, G. M. Gastrorenal axis. Hypertension 67, 1056–1063 (2016).
Bankir, L., Roussel, R. & Bouby, N. Protein- and diabetes-induced glomerular hyperfiltration: role of glucagon, vasopressin, and urea. Am. J. Physiol. Renal Physiol. 309, F2–F23 (2015).
Tonneijck, L. et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J. Am. Soc. Nephrol. 28, 1023–1039 (2017). In-depth review of the hitherto proposed mechanisms involved in diabetes-related glomerular hyperfiltration, its clinical significance and available and emerging interventions that might attenuate this renal haemodynamic abnormality.
Kutina, A. V., Golosova, D. V., Marina, A. S., Shakhmatova, E. I. & Natochin, Y. V. Role of vasopressin in the regulation of renal sodium excretion: interaction with glucagon-like peptide-1. J Neuroendocrinol. http://dx.doi.org/10.1111/jne.12367 (2016).
Marina, A. S., Kutina, A. V., Shakhmatoba, E. I. & Natochin, Y. V. Involvement of glucagon-like peptide-1 in the regulation of selective excretion of sodium or chloride ions by the kidneys. Bull. Exp. Biol. Med. 162, 436–440 (2017).
Marina, A. S., Kutina, A. V., Shakhmatova, E. I., Balbotkina, E. V. & Natochin, Y. V. Stimulation of glucagon-like peptide-1 secretion by water loading in human. Dokl. Biol. Sci. 459, 323–325 (2014).
McKay, N. J., Galante, D. L. & Daniels, D. Endogenous glucagon-like peptide-1 reduces drinking behavior and is differentially engaged by water and food intakes in rats. J. Neurosci. 34, 16417–16423 (2014).
Petersen, B., Christiansen, J. & Holst, J. J. A glucose-dependent mechanism in jejunum inhibits gastric acid secretion: a response mediated through enteroglucagon? Scand. J. Gastroenterol. 20, 193–197 (1985).
Navarro, M. et al. Colocalization of glucagon-like peptide-1 (GLP-1) receptors, glucose transporter GLUT-2, and glucokinase mRNAs in rat hypothalamic cells: evidence for a role of GLP-1 receptor agonists as an inhibitory signal for food and water intake. J. Neurochem. 67, 1982–1991 (1996).
Tang-Christensen, M. et al. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am. J. Physiol. 271, R848–R856 (1996).
Gutzwiller, J. P. et al. Glucagon-like peptide-1 is involved in sodium and water homeostasis in humans. Digestion 73, 142–150 (2006).
McKay, N. J., Kanoski, S. E., Hayes, M. R. & Daniels, D. Glucagon-like peptide-1 receptor agonists suppress water intake independent of effects on food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1755–R1764 (2011).
McKay, N. J. & Daniels, D. Glucagon-like peptide-1 receptor agonist administration suppresses both water and saline intake in rats. J. Neuroendocrinol. 25, 929–938 (2013).
Crajoinas, R. O. et al. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am. J. Physiol. Renal Physiol. 301, F355–F363 (2011).
Fujita, H. et al. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. 85, 579–589 (2014).
Jensen, E. P. et al. Activation of GLP-1 receptors on vascular smooth muscle cells reduces the autoregulatory response in afferent arterioles and increases renal blood flow. Am. J. Physiol. Renal Physiol. 308, F867–F877 (2015).
Kodera, R. et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 54, 965–978 (2011).
Korner, M., Stockli, M., Waser, B. & Reubi, J. C. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J. Nucl. Med. 48, 736–743 (2007).
Pyke, C. et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 155, 1280–1290 (2014). Study that gives important insight into the molecular mode of action of GLP-1 by identifying the cellular localization of GLP-1R in monkey and human tissue using the most extensively validated monoclonal antibody to date.
Schlatter, P., Beglinger, C., Drewe, J. & Gutmann, H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul. Pept. 141, 120–128 (2007).
Kirino, Y. et al. Interrelationship of dipeptidyl peptidase IV (DPP4) with the development of diabetes, dyslipidaemia and nephropathy: a streptozotocin-induced model using wild-type and DPP4-deficient rats. J. Endocrinol. 200, 53–61 (2009).
Skov, J. et al. Glucagon-like peptide-1 (GLP-1): effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J. Clin. Endocrinol. Metab. 98, E664–E671 (2013).
Gutzwiller, J. P. et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J. Clin. Endocrinol. Metab. 89, 3055–3061 (2004). Clinical study demonstrating that acute GLP-1 infusion reduces glomerular hyperfiltration and produces an increase in urinary sodium excretion and a decrease in urinary hydrogen excretion in insulin-resistant obese men.
Farah, L. X. et al. The physiological role of glucagon-like peptide-1 in the regulation of renal function. Am. J. Physiol. Renal Physiol. 310, F123–F127 (2016).
Muskiet, M. H. et al. Acute renal haemodynamic effects of glucagon-like peptide-1 receptor agonist exenatide in healthy overweight men. Diabetes Obes. Metab. 18, 178–185 (2016).
Tonneijck, L. et al. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double-blind, placebo-controlled trial. Diabetologia 59, 1412–1421 (2016).
Skov, J. et al. Short-term effects of liraglutide on kidney function and vasoactive hormones in type 2 diabetes: a randomized clinical trial. Diabetes Obes. Metab. 18, 581–589 (2016).
Lovshin, J. A. et al. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care 38, 132–139 (2015).
Tonneijck, L. et al. Renal effects of dpp-4 inhibitor sitagliptin or glp-1 receptor agonist liraglutide in overweight patients with type 2 diabetes: a 12-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 39, 2042–2050 (2016).
Tonneijck, L. et al. Postprandial renal haemodynamic effect of lixisenatide versus once-daily insulin glulisine in type 2 diabetes patients on insulin-glargine: an 8-week, randomised, open-label trial. Diabetes Obes. Metab. http://dx.doi.org/10.1111/dom.12985 (2017).
Zhou, X. et al. Acute hemodynamic and renal effects of glucagon-like peptide 1 analog and dipeptidyl peptidase-4 inhibitor in rats. Cardiovasc. Diabetol. 14, 29 (2015).
Takashima, S. et al. Stromal cell-derived factor-1 is upregulated by dipeptidyl peptidase-4 inhibition and has protective roles in progressive diabetic nephropathy. Kidney Int. 90, 783–796 (2016).
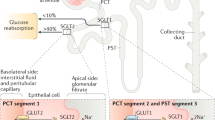
Lovshin, J. A. et al. Dipeptidyl peptidase-4 inhibition stimulates distal tubular natriuresis and increases in circulating SDF-1alpha1-67 in patients with type 2 diabetes. Diabetes Care 40, 1073–1081 (2017). Clinical study suggesting that DPP-4 inhibition blocks distal, rather than proximal, sodium reabsorption after prolonged therapy in patients with T2DM, potentially as a result of decreased inactivation of SDF1 α rather than a mechanism involving GLP-1.
Girardi, A. C., Fukuda, L. E., Rossoni, L. V., Malnic, G. & Reboucas, N. A. Dipeptidyl peptidase IV inhibition downregulates Na+-H+ exchanger NHE3 in rat renal proximal tubule. Am. J. Physiol. Renal Physiol. 294, F414–F422 (2008).
Girardi, A. C., Knauf, F., Demuth, H. U. & Aronson, P. S. Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am. J. Physiol. Cell Physiol. 287, C1238–C1245 (2004).
Carraro-Lacroix, L. R., Malnic, G. & Girardi, A. C. Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 297, F1647–F1655 (2009).
Moreno, C., Mistry, M. & Roman, R. J. Renal effects of glucagon-like peptide in rats. Eur. J. Pharmacol. 434, 163–167 (2002).
McDonough, A. A., Leong, P. K. & Yang, L. E. Mechanisms of pressure natriuresis: how blood pressure regulates renal sodium transport. Ann. NY Acad. Sci. 986, 669–677 (2003).
Rieg, T. et al. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am. J. Physiol. Renal Physiol. 303, F963–F971 (2012).
Tonneijck, L., Muskiet, M. H., Smits, M. M., van Raalte, D. H. & Diamant, M. Combining incretin-based drugs and RAAS inhibitors: more cons than pros? Lancet Diabetes Endocrinol. 2, 684–685 (2014).
Asmar, A. et al. Renal extraction and acute effects of glucagon-like peptide-1 on central and renal hemodynamics in healthy men. Am. J. Physiol. Endocrinol. Metab. 308, E641–E649 (2015).
Asmar, A. et al. Glucagon-like peptide-1 does not have acute effects on central or renal hemodynamics in patients with type 2 diabetes without nephropathy. Am. J. Physiol. Endocrinol. Metab. 310, E744–E753 (2016).
Kim, M. et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat. Med. 19, 567–575 (2013).
Skov, J., Holst, J. J., Gotze, J. P., Frokiaer, J. & Christiansen, J. S. Glucagon-like peptide-1: effect on pro-atrial natriuretic peptide in healthy males. Endocr. Connect. 3, 11–16 (2014).
Marina, A. S., Kutina, A. V. & Natochin, Y. V. Exenatide stimulates solute-free water clearance by the rat kidney in hyperhydration. Dokl. Biol. Sci. 437, 85–87 (2011).
Kutina, A. V., Marina, A. S., Shakhmatova, E. I. & Natochin, Y. V. Physiological mechanisms for the increase in renal solute-free water clearance by a glucagon-like peptide-1 mimetic. Clin. Exp. Pharmacol. Physiol. 40, 510–517 (2013).
Shakhmatova, E. I. et al. Exenatide stimulated solute-free water excretion by human kidney [Russian]. Ross. Fiziol. Zh. Im. I. M. Sechenova 98, 1021–1029 (2012).
Thomson, S. C., Kashkouli, A. & Singh, P. Glucagon-like peptide-1 receptor stimulation increases GFR and suppresses proximal reabsorption in the rat. Am. J. Physiol. Renal Physiol. 304, F137–F144 (2013).
Thomson, S. C. et al. Temporal adjustment of the juxtaglomerular apparatus during sustained inhibition of proximal reabsorption. J. Clin. Invest. 104, 1149–1158 (1999).
von Scholten, B. J., Hansen, T. W., Goetze, J. P., Persson, F. & Rossing, P. Glucagon-like peptide 1 receptor agonist (GLP-1 RA): long-term effect on kidney function in patients with type 2 diabetes. J. Diabetes Complications 29, 670–674 (2015).
von Scholten, B. J., Lajer, M., Goetze, J. P., Persson, F. & Rossing, P. Time course and mechanisms of the anti-hypertensive and renal effects of liraglutide treatment. Diabet. Med. 32, 343–352 (2015).
von Scholten, B. J. et al. The effect of liraglutide on renal function: a randomized clinical trial. Diabetes Obes. Metab. 19, 239–247 (2017). Placebo-controlled cross-over trial in patients with T2DM and albuminuria that shows that liraglutide therapy reduces 24-h urinary albumin excretion by lowering blood pressure, rather than by improving HbA 1c levels and body weight.
Tonneijck, L., Smits, M. M., van Raalte, D. H. & Muskiet, M. H. Incretin-based drugs and renoprotection-is hyperfiltration key? Kidney Int. 87, 660–661 (2015).
Cherney, D. Z. et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129, 587–597 (2014). First description in humans of the ability of SGLT2 inhibitors to attenuate glomerular hyperfiltration, likely by affecting tubuloglomerular feedback mechanisms.
Park, C. W. et al. Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J. Am. Soc. Nephrol. 18, 1227–1238 (2007).
Arjona Ferreira, J. C. et al. Efficacy and safety of sitagliptin versus glipizide in patients with type 2 diabetes and moderate-to-severe chronic renal insufficiency. Diabetes Care 36, 1067–1073 (2013).
Davies, M. J. et al. Efficacy and safety of liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA-RENAL): a randomized clinical trial. Diabetes Care 39, 222–230 (2016).
Groop, P. H. et al. Linagliptin treatment in subjects with type 2 diabetes with and without mild-to-moderate renal impairment. Diabetes Obes. Metab. 16, 560–568 (2014).
McGill, J. B. et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care 36, 237–244 (2013).
Cooper, M. E. et al. Kidney disease end points in a pooled analysis of individual patient-level data from a large clinical trials program of the dipeptidyl peptidase 4 inhibitor linagliptin in type 2 diabetes. Am. J. Kidney Dis. 66, 441–449 (2015).
Tuttle, K. R., Heilmann, C., Hoogwerf, B. J., Brown, C. & Anderson, P. W. Effects of exenatide on kidney function, adverse events, and clinical end points of kidney disease in type 2 diabetes. Am. J. Kidney Dis. 62, 396–398 (2013).
Tuttle, K. R. et al. Effects of once-weekly dulaglutide on kidney function in patients with type 2 diabetes in phase II and III clinical trials. Diabetes Obes. Metab. 19, 436–441 (2017). Analysis of pooled phase II and phase III RCTs that describes the albuminuria-lowering effect of dulaglutide compared to insulin glargine and other active comparators beyond glycaemic control.
Ott, C. et al. Effects of linagliptin on renal endothelial function in patients with type 2 diabetes: a randomised clinical trial. Diabetologia 59, 2579–2587 (2016).
Gaede, P. et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. 348, 383–393 (2003).
Oellgaard, J. et al. Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long-term renal benefits. Kidney Int. 91, 982–988 (2017). Key post hoc analysis of the landmark Steno-2 trial showing that intensified, multifactorial treatment slows progression of diabetic nephropathy and renal function loss in microalbuminuric patients with T2DM.
Ali, M. K. et al. Achievement of goals in U.S. diabetes care, 1999–2010. N. Engl. J. Med. 368, 1613–1624 (2013).
Liu, S. C., Tu, Y. K., Chien, M. N. & Chien, K. L. Effect of antidiabetic agents added to metformin on glycaemic control, hypoglycaemia and weight change in patients with type 2 diabetes: a network meta-analysis. Diabetes Obes. Metab. 14, 810–820 (2012).
Sun, F. et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss in patients with type 2 diabetes: a systematic review and network meta-analysis. J. Diabetes Res. 2015, 157201 (2015).
Pi-Sunyer, X. et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 373, 11–22 (2015).
European Medicines Agency. Saxenda. EMA http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003780/human_med_001855.jsp&mid=WC0b01ac058001d124 (2017).
Bunck, M. C. et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 33, 1734–1737 (2010).
Jendle, J. et al. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes. Metab. 11, 1163–1172 (2009).
Edwards, C. M., Todd, J. F., Ghatei, M. A. & Bloom, S. R. Subcutaneous glucagon-like peptide-1 (7–36) amide is insulinotropic and can cause hypoglycaemia in fasted healthy subjects. Clin. Sci. (Lond.) 95, 719–724 (1998).
Halbirk, M. et al. Cardiovascular and metabolic effects of 48-h glucagon-like peptide-1 infusion in compensated chronic patients with heart failure. Am. J. Physiol. Heart Circ. Physiol. 298, H1096–H1102 (2010).
Sun, F. et al. Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Res. Clin. Pract. 110, 26–37 (2015).
Zhang, X. & Zhao, Q. Effects of dipeptidyl peptidase-4 inhibitors on blood pressure in patients with type 2 diabetes: a systematic review and meta-analysis. J. Hypertens. 34, 167–175 (2016).
Davies, M. J. et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 314, 687–699 (2015). Study that establishes the efficacy and safety of liraglutide in patients with T2DM and moderate renal impairment, but shows a lack of effect of acute and prolonged therapy with this GLP-1R agonist on eGFR trajectory compared to placebo.
Sun, F. et al. Effect of glucagon-like peptide-1 receptor agonists on lipid profiles among type 2 diabetes: a systematic review and network meta-analysis. Clin. Ther. 37, 225–241.e8 (2015).
Monami, M., Lamanna, C., Desideri, C. M. & Mannucci, E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv. Ther. 29, 14–25 (2012).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016). Authoritative review describing how GLP-1 and clinically approved GLP-1R agonists engage mechanisms that influence the risk of developing cardiovascular disease in diabetes and obesity.
Smits, M. M. et al. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo-controlled trial. Diabetologia 59, 2588–2593 (2016).
Kooijman, S. et al. Central GLP-1 receptor signalling accelerates plasma clearance of triacylglycerol and glucose by activating brown adipose tissue in mice. Diabetologia 58, 2637–2646 (2015).
Alter, M. L. et al. DPP-4 inhibition on top of angiotensin receptor blockade offers a new therapeutic approach for diabetic nephropathy. Kidney Blood Press. Res. 36, 119–130 (2012).
Brosius, F. C. et al. Mouse models of diabetic nephropathy. J. Am. Soc. Nephrol. 20, 2503–2512 (2009).
Nakagawa, T. et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J. Am. Soc. Nephrol. 18, 539–550 (2007).
Jung, E., Kim, J., Ho Kim, S., Kim, S. & Cho, M. H. Gemigliptin improves renal function and attenuates podocyte injury in mice with diabetic nephropathy. Eur. J. Pharmacol. 761, 116–124 (2015).
Jung, G. S. et al. Renoprotective effect of gemigliptin, a dipeptidyl peptidase-4 inhibitor, in streptozotocin-induced type 1 diabetic mice. Diabetes Metab. J. 40, 211–221 (2016).
Kodera, R. et al. Dipeptidyl peptidase-4 inhibitor ameliorates early renal injury through its anti-inflammatory action in a rat model of type 1 diabetes. Biochem. Biophys. Res. Commun. 443, 828–833 (2014).
Liu, W. J. et al. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J. Pharmacol. Exp. Ther. 340, 248–255 (2012).
Wang, Y. et al. Attenuation of renovascular damage in zucker diabetic fatty rat by NWT-03, an egg protein hydrolysate with ACE- and DPP4-inhibitory activity. PLoS ONE 7, e46781 (2012).
Zhou, S. J. et al. Liraglutide ameliorates renal injury in streptozotocininduced diabetic rats by activating endothelial nitric oxide synthase activity via the downregulation of the nuclear factor-κB pathway. Mol. Med. Rep. 10, 2587–2594 (2014).
Hendarto, H. et al. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism 61, 1422–1434 (2012).
Li, W. et al. Inhibition of the expression of TGF-beta1 and CTGF in human mesangial cells by exendin-4, a glucagon-like peptide-1 receptor agonist. Cell. Physiol. Biochem. 30, 749–757 (2012).
Sourris, K. C. et al. Can targeting the incretin pathway dampen RAGE-mediated events in diabetic nephropathy? Curr. Drug Targets 17, 1252–1264 (2016).
Ishibashi, Y. et al. Glucagon-like peptide-1 inhibits angiotensin II-induced mesangial cell damage via protein kinase A. Microvasc. Res. 84, 395–398 (2012).
Mima, A. et al. Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCβ activation in diabetes. Diabetes 61, 2967–2979 (2012).
Nistala, R. et al. DPP4 inhibition attenuates filtration barrier injury and oxidant stress in the zucker obese rat. Obesity (Silver Spring) 22, 2172–2179 (2014).
Kanasaki, K. et al. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes 63, 2120–2131 (2014). Preclinical study in mice with streptozotocin-induced diabetes that showed glucose-independent amelioration of kidney fibrosis with the DPP-4 inhibitor linagliptin.
Shi, S. et al. Interactions of DPP-4 and integrin beta1 influences endothelial-to-mesenchymal transition. Kidney Int. 88, 479–489 (2015).
Panchapakesan, U. & Pollock, C. A. DPP-4 inhibitors-renoprotection in diabetic nephropathy? Diabetes 63, 1829–1830 (2014).
Zeisberg, M. & Zeisberg, E. M. Evidence for antifibrotic incretin-independent effects of the DPP-4 inhibitor linagliptin. Kidney Int. 88, 429–431 (2015).
Derosa, G. et al. Exenatide versus glibenclamide in patients with diabetes. Diabetes Technol. Ther. 12, 233–240 (2010).
Rizzo, M. et al. Liraglutide reduces oxidative stress and restores heme oxygenase-1 and ghrelin levels in patients with type 2 diabetes: a prospective pilot study. J. Clin. Endocrinol. Metab. 100, 603–606 (2015).
Zhang, H., Zhang, X., Hu, C. & Lu, W. Exenatide reduces urinary transforming growth factor-beta1 and type IV collagen excretion in patients with type 2 diabetes and microalbuminuria. Kidney Blood Press. Res. 35, 483–488 (2012).
Imamura, S., Hirai, K. & Hirai, A. The glucagon-like peptide-1 receptor agonist, liraglutide, attenuates the progression of overt diabetic nephropathy in type 2 diabetic patients. Tohoku J. Exp. Med. 231, 57–61 (2013).
Zavattaro, M. et al. One-year treatment with liraglutide improved renal function in patients with type 2 diabetes: a pilot prospective study. Endocrine 50, 620–626 (2015).
Pawaskar, M., Tuttle, K. R., Li, Q., Best, J. H. & Anderson, P. W. Observational study of kidney function and albuminuria in patients with type 2 diabetes treated with exenatide BID versus insulin glargine. Ann. Pharmacother. 48, 571–576 (2014).
Tuttle, K. R. et al. Dulaglutide vs. glargine, both combined with lispro, mitigated eGFR decline in people with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7). Presented at ADA's 77th Scientific Sessions, San Diego, California, USA. Poster number: 142-LB (2017).
Zheng, T. et al. Increased plasma dipeptidyl peptidase-4 activities are associated with high prevalence of diabetic nephropathy in Chinese patients with newly diagnosed type 2 diabetes: a cross-sectional study. Diab. Vasc. Dis. Res. 13, 127–136 (2016).
Duvnjak, L., Perkovic, M. N. & Blaslov, K. Dipeptidyl peptidase-4 activity is associated with urine albumin excretion in type 1 diabetes. J. Diabetes Complications 31, 218–222 (2017).
Zheng, T., Baskota, A., Gao, Y., Tian, H. & Yang, F. Increased plasma dipeptidyl peptidase 4 activities predict new-onset microalbuminuria in association with its proinflammatory effects in Chinese without diabetes: a four-year prospective study. Nephrol. Dial. Transplant. 30, 460–466 (2015).
Sun, A. L. et al. Dipeptidyl peptidase-IV is a potential molecular biomarker in diabetic kidney disease. Diab. Vasc. Dis. Res. 9, 301–308 (2012).
Mitic, B., Lazarevic, G., Vlahovic, P., Rajic, M. & Stefanovic, V. Diagnostic value of the aminopeptidase N, N-acetyl-beta-D-glucosaminidase and dipeptidylpeptidase IV in evaluating tubular dysfunction in patients with glomerulopathies. Ren. Fail. 30, 896–903 (2008).
Shi, S., Koya, D. & Kanasaki, K. Dipeptidyl peptidase-4 and kidney fibrosis in diabetes. Fibrogen. Tissue Repair 9, 1 (2016).
Sakata, K. et al. Efficacy of alogliptin, a dipeptidyl peptidase-4 inhibitor, on glucose parameters, the activity of the advanced glycation end product (AGE) - receptor for AGE (RAGE) axis and albuminuria in Japanese type 2 diabetes. Diabetes Metab. Res. Rev. 29, 624–630 (2013).
Hattori, S. Sitagliptin reduces albuminuria in patients with type 2 diabetes. Endocr. J. 58, 69–73 (2011).
Iazzetta, N. et al. Nephroprotection with saxagliptin. G. Ital. Nefrol. 32, gin/32.6.11 (2015).
Kim, Y. G. et al. Renal protective effect of dpp-4 inhibitors in type 2 diabetes mellitus patients: a cohort study. J. Diabetes Res. 2016, 1423191 (2016).
Groop, P. H. et al. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care 36, 3460–3468 (2013).
Groop, P. H. et al. Linagliptin and its effects on hyperglycaemia and albuminuria in patients with type 2 diabetes and renal dysfunction: the randomized MARLINA-T2D trial. Diabetes Obes. Metab. http://dx.doi.org/10.1111/dom.13041 (2017). This sufficiently powered clinical study did not find a beneficial effect of linagliptin versus placebo on albuminuria in patients with T2DM, although exploratory analyses indicated more responders and fewer non-responders in terms of UACR in the linagliptin group.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01897532 (2017).
Mori, H., Okada, Y., Arao, T. & Tanaka, Y. Sitagliptin improves albuminuria in patients with type 2 diabetes mellitus. J. Diabetes Investig. 5, 313–319 (2014).
Bergenstal, R. M. et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 376, 431–439 (2010).
Food and Drug Administration. Guidance for industry: diabetes mellitus — evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. FDA http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf (2008).
Pfeffer, M. A., Claggett, B. & Probstfield, J. L. Lixisenatide in type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 374, 1095–1096 (2016). The first cardiovascular safety trial of a GLP-1R agonist, demonstrating that lixisenatide does not augment MACE in patients with T2DM who had a recent acute coronary event.
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016). Second cardiovascular safety trial to demonstrate that a GLP-1R agonist reduces MACE and microvascular (including renal) events in patients with T2DM and high cardiovascular risk.
Muskiet, M. H., Tonneijck, L., van Bommel, E. J., Smits, M. M. & van Raalte, D. H. Renoprotection in LEADER and EMPA-REG OUTCOME. Lancet Diabetes Endocrinol. 4, 812–814 (2016).
Scirica, B. M. et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369, 1317–1326 (2013). First cardiovascular safety trial of a DPP-4 inhibitor in patients with T2DM and high cardiovascular risk, showing that saxagliptin does not augment MACE but increases hospitalization for heart failure.
White, W. B. et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 369, 1327–1335 (2013). Cardiovascular safety trial demonstrating that the DPP-4 inhibitor alogliptin does not increase MACE in patients with T2DM who had a recent acute coronary event.
Green, J. B. et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 373, 232–242 (2015). Cardiovascular safety trial demonstrating that the DPP-4 inhibitor sitagliptin does not increase MACE or hospitalization for heart failure in patients with T2DM and high cardiovascular risk.
Kongwatcharapong, J., Dilokthornsakul, P., Nathisuwan, S., Phrommintikul, A. & Chaiyakunapruk, N. Effect of dipeptidyl peptidase-4 inhibitors on heart failure: a meta-analysis of randomized clinical trials. Int. J. Cardiol. 211, 88–95 (2016).
Scirica, B. M. et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation 130, 1579–1588 (2014).
Muskiet, M. H. et al. Regarding article, “Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial”. Circulation 132, e120 (2015).
Scirica, B. M. et al. Response to letter regarding article, “Heart failure, saxagliptin and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial”. Circulation 132, e121–e122 (2015).
White, W. B. et al. Angiotensin-converting enzyme inhibitor use and major cardiovascular outcomes in type 2 diabetes mellitus treated with the dipeptidyl peptidase 4 inhibitor alogliptin. Hypertension 68, 606–613 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02130687 (2016).
Mosenzon, O. et al. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care 40, 69–76 (2017). Secondary analysis of renal end points in a cardiovascular safety trial of a DPP-4 inhibitor in patients with T2DM at high cardiovascular risk, showing that saxagliptin reduces albuminuria in a glucose-independent manner.
Cornel, J. H. et al. Effect of sitagliptin on kidney function and respective cardiovascular outcomes in type 2 diabetes: outcomes from TECOS. Diabetes Care 39, 2304–2310 (2016).
Nauck, M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes. Metab. 18, 203–216 (2016).
Storgaard, H., Cold, F., Gluud, L. L., Vilsboll, T. & Knop, F. K. Glucagon-like peptide-1 agonists and risk of acute pancreatitis in patients with type 2 diabetes. Diabetes Obes. Metab. http://dx.doi.org/10.1111/dom.12885 (2017).
Abbas, A. S., Dehbi, H. M. & Ray, K. K. Cardiovascular and non-cardiovascular safety of dipeptidyl peptidase-4 inhibition: a meta-analysis of randomized controlled cardiovascular outcome trials. Diabetes Obes. Metab. 18, 295–299 (2016).
Azoulay, L. et al. Incretin based drugs and the risk of pancreatic cancer: international multicentre cohort study. BMJ 352, i581 (2016).
Shih, C. J. et al. Association between use of dipeptidyl peptidase-4 inhibitors and the risk of acute kidney injury: a nested case-control study. Mayo Clin. Proc. 91, 867–872 (2016).
Pendergrass, M., Fenton, C., Haffner, S. M. & Chen, W. Exenatide and sitagliptin are not associated with increased risk of acute renal failure: a retrospective claims analysis. Diabetes Obes. Metab. 14, 596–600 (2012).
Chang, M. W. et al. Sitagliptin protects rat kidneys from acute ischemia-reperfusion injury via upregulation of GLP-1 and GLP-1 receptors. Acta Pharmacol. Sin. 36, 119–130 (2015).
Chen, Y. T. et al. Exendin-4 and sitagliptin protect kidney from ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J. Transl Med. 11, 270 (2013).
Glorie, L. L. et al. DPP4 inhibition improves functional outcome after renal ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 303, F681–F688 (2012).
Nuransoy, A. et al. Protective effect of sitagliptin against renal ischemia reperfusion injury in rats. Ren. Fail. 37, 687–693 (2015).
Vaghasiya, J., Sheth, N., Bhalodia, Y. & Manek, R. Sitagliptin protects renal ischemia reperfusion induced renal damage in diabetes. Regul. Pept. 166, 48–54 (2011).
Youssef, M. I., Mahmoud, A. A. & Abdelghany, R. H. A new combination of sitagliptin and furosemide protects against remote myocardial injury induced by renal ischemia/reperfusion in rats. Biochem. Pharmacol. 96, 20–29 (2015).
Yang, H. et al. Exendin-4 ameliorates renal ischemia-reperfusion injury in the rat. J. Surg. Res. 185, 825–832 (2013).
Chao, C. T., Wang, J., Wu, H. Y., Chien, K. L. & Hung, K. Y. Dipeptidyl peptidase 4 inhibitor use is associated with a lower risk of incident acute kidney injury in patients with diabetes. Oncotarget http://dx.doi.org/10.18632/oncotarget.18081 (2017).
Keller, J. et al. Effect of exenatide on cholecystokinin-induced gallbladder emptying in fasting healthy subjects. Regul. Pept. 179, 77–83 (2012).
Smits, M. M. et al. Biliary effects of liraglutide and sitagliptin, a 12-week randomized placebo-controlled trial in type 2 diabetes patients. Diabetes Obes. Metab. 18, 1217–1225 (2016).
[No authors listed.] Early worsening of diabetic retinopathy in the diabetes control and complications trial. Arch. Ophthalmol. 116, 874–886 (1998).
Ipp, E., Genter, P. & Childress, K. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 376, 890–891 (2017).
Vilsboll, T. Cardiovascular outcomes with semaglutide in subjects with type 2 diabetes mellitus (SUSTAIN 6). 77 th American Diabetes Association 2017 Scientific Sessions. June 9, San Diego, California. Presentation 1-AC-SY09 (2017).
Cherney, D. Z. I. et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 5, 610–621 (2017).
Muskiet, M. H. A., Heerspink, H. J. L. & van Raalte, D. H. SGLT2 inhibition: a new era in renoprotective medicine? Lancet Diabetes Endocrinol. 5, 569–571 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01243424 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01794143 (2017).
Ferrannini, G. et al. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care 38, 1730–1735 (2015).
Frias, J. P. et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 4, 1004–1016 (2016). First clinical trial to investigate the efficacy and safety of combined SGLT2 inhibitor and GLP-1R agonist therapy in patients with T2DM, suggesting additive or synergistic effects on weight loss.
Nauck, M. A. & Meier, J. J. GLP-1 receptor agonists and SGLT2 inhibitors: a couple at last? Lancet Diabetes Endocrinol. 4, 963–964 (2016).
Jackson, E. K., Kochanek, S. J. & Gillespie, D. G. Dipeptidyl peptidase IV regulates proliferation of preglomerular vascular smooth muscle and mesangial cells. Hypertension 60, 757–764 (2012).
Kettmann, U., Humbel, B. & Holzhausen, H. J. Ultrastructural localization of dipeptidylpeptidase IV in the glomerulum of the rat kidney. Acta Histochem. 92, 225–227 (1992).
Lee, J. W., Chou, C. L. & Knepper, M. A. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J. Am. Soc. Nephrol. 26, 2669–2677 (2015).
European Medicines Agency. European public assessment reports. EMA http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124 (2017).
Nauck, M. A. et al. A phase 2, randomized, dose-finding study of the novel once-weekly human glp-1 analog, semaglutide, compared with placebo and open-label liraglutide in patients with type 2 diabetes. Diabetes Care 39, 231–241 (2016).
Aaboe, K. et al. Twelve weeks treatment with the DPP-4 inhibitor, sitagliptin, prevents degradation of peptide YY and improves glucose and non-glucose induced insulin secretion in patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 12, 323–333 (2010).
Ballantyne, G. H. Peptide YY(1–36) and peptide YY(3–36): part i. distribution, release and actions. Obes. Surg. 16, 651–658 (2006).
Nystrom, T., Gonon, A. T., Sjoholm, A. & Pernow, J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul. Pept. 125, 173–177 (2005).
Nystrom, T. et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am. J. Physiol. Endocrinol. Metab. 287, E1209–E1215 (2004).
Lovshin, J. A. & Zinman, B. Blood pressure-lowering effects of incretin-based diabetes therapies. Can. J. Diabetes 38, 364–371 (2014).
Girardi, A. C., Degray, B. C., Nagy, T., Biemesderfer, D. & Aronson, P. S. Association of Na+-H+ exchanger isoform NHE3 and dipeptidyl peptidase IV in the renal proximal tubule. J. Biol. Chem. 276, 46671–46677 (2001).
Vanderheyden, M. et al. Dipeptidyl-peptidase IV and B-type natriuretic peptide. from bench to bedside. Clin. Chem. Lab. Med. 47, 248–252 (2009).
Sato, Y. et al. Role of dipeptidyl peptidase IV (DPP4) in the development of dyslipidemia: DPP4 contributes to the steroid metabolism pathway. Life Sci. 88, 43–49 (2011).
Ishibashi, Y., Nishino, Y., Matsui, T., Takeuchi, M. & Yamagishi, S. Glucagon-like peptide-1 suppresses advanced glycation end product-induced monocyte chemoattractant protein-1 expression in mesangial cells by reducing advanced glycation end product receptor level. Metabolism 60, 1271–1277 (2011).
Ojima, A. et al. Glucagon-like peptide-1 receptor agonist inhibits asymmetric dimethylarginine generation in the kidney of streptozotocin-induced diabetic rats by blocking advanced glycation end product-induced protein arginine methyltranferase-1 expression. Am. J. Pathol. 182, 132–141 (2013).
Camara, N. O., Iseki, K., Kramer, H., Liu, Z. H. & Sharma, K. Kidney disease and obesity: epidemiology, mechanisms and treatment. Nat. Rev. Nephrol. 13, 181–190 (2017).
Fadini, G. P. et al. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care 33, 1607–1609 (2010).
Fujita, H. et al. DPP-4 inhibition with alogliptin on top of angiotensin II type 1 receptor blockade ameliorates albuminuria via up-regulation of SDF-1alpha in type 2 diabetic patients with incipient nephropathy. Endocr. J. 61, 159–166 (2014).
Jelsing, J. et al. Liraglutide: short-lived effect on gastric emptying — long lasting effects on body weight. Diabetes Obes. Metab. 14, 531–538 (2012).
Shi, S., Kanasaki, K. & Koya, D. Linagliptin but not sitagliptin inhibited transforming growth factor-beta2-induced endothelial DPP-4 activity and the endothelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 471, 184–190 (2016).
Author information
Authors and Affiliations
Contributions
M.H.A.M., L.T., M.M.S., J.A.J. and D.H.vR. researched the data, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission. M.J.B.vB., M.H.H.K. and E.J.H. made substantial contributions to discussions of the content and reviewed and/or edited the manuscript before submission. M.H.A.M. and L.T. contributed equally to this Review.
Corresponding author
Ethics declarations
Competing interests
Through M.H.H.K., the VU University Medical Center has received research grants from AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Sanofi. M.H.A.M. and L.T. consulted for Eli Lilly & Co., and D.H.vR. serves on advisory boards of Sanofi Aventis and Merck Sharp & Dohme (all honoraria paid to employer). The other authors declare no competing interests.
Supplementary information
Supplementary information
Supplementary Figures and Tables (PDF 594 kb)
Rights and permissions
About this article
Cite this article
Muskiet, M., Tonneijck, L., Smits, M. et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol 13, 605–628 (2017). https://doi.org/10.1038/nrneph.2017.123
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.123
This article is cited by
-
Combination therapy for kidney disease in people with diabetes mellitus
Nature Reviews Nephrology (2024)
-
Systemerkrankung Herzinsuffizienz
Die Kardiologie (2024)
-
In vivo mapping of hemodynamic responses mediated by tubuloglomerular feedback in hypertensive kidneys
Scientific Reports (2023)
-
Glucagon-Like Peptide-1 Receptor Agonist and Risk of Diabetic Retinopathy in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis of Randomized Placebo-Controlled Trials
Clinical Drug Investigation (2023)
-
Hypertension in diabetes
Pediatric Nephrology (2023)