Key Points
-
Diabetic nephropathy has severe individual and societal consequences, owing to its high morbidity, mortality and health-care costs
-
Despite the availability of anti-glycaemic, renoprotective and antihypertensive agents, diabetes mellitus remains the most common cause of end-stage renal disease in developed countries
-
New biomarkers that can identify patients at risk of diabetic nephropathy are needed, as well as new agents that directly target the pathogenic pathways of diabetic nephropathy
-
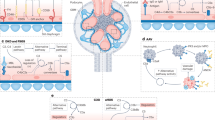
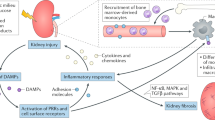
Growing evidence suggests that the complement system has a pathogenic role in the development of diabetic nephropathy
-
Mannose-binding protein is a strong biomarker of diabetic nephropathy in patients with type 1 diabetes mellitus (T1DM) and T2DM; H-ficolin might be useful to identify patients with T1DM at risk of persistent microalbuminuria
-
Inhibiting specific components of the complement system might be an effective therapeutic strategy to treat diabetic nephropathy
Abstract
The development of type 1 and type 2 diabetes mellitus has a substantial negative impact on morbidity and mortality and is responsible for substantial individual and socioeconomic costs worldwide. One of the most serious consequences of diabetes mellitus is the development of diabetic angiopathy, which manifests clinically as microvascular and macrovascular complications. One microvascular complication, diabetic nephropathy, is the most common cause of end-stage renal disease in developed countries. Although several available therapeutic interventions can delay the onset and progression of diabetic nephropathy, morbidity associated with this disease remains high and new therapeutic approaches are needed. In addition, not all patients with diabetes mellitus will develop diabetic nephropathy and thus new biomarkers are needed to identify individuals who will develop this life-threatening disease. An increasing body of evidence points toward a role of the complement system in the pathogenesis of diabetic nephropathy. For example, circulating levels of mannose-binding lectin (MBL), a pattern recognition molecule of the innate immune system, have emerged as a robust biomarker for the development and progression of this disease, and evidence suggests that MBL, H-ficolin, complement component C3 and the membrane attack complex might contribute to renal injury in the hyperglycaemic mileu. New approaches to modulate the complement system might lead to the development of new agents to prevent or slow the progression of diabetic nephropathy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
International Diabetes Federation. IDF Diabetes Atlas, Seventh Edition. Diabetes Atlas http://www.diabetesatlas.org/resources/2015-atlas.html (2015).
Schrijvers, B. F., De Vriese, A. S. & Flyvbjerg, A. From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr. Rev. 25, 971–1010 (2004).
Pfützner, A. & Forst, T. High-sensitivity C-reactive protein as cardiovascular risk marker in patients with diabetes mellitus. Diabetes Technol. Ther. 8, 28–36 (2006).
Schalkwijk, C. G. et al. Plasma concentration of C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia 42, 351–357 (1999).
Flyvbjerg, A. Diabetic angiopathy, the complement system and the tumour necrosis factor superfamily. Nat. Rev. Endocrinol. 6, 94–101 (2010).
Wada, J. & Makino, H. Innate immunity in diabetes and diabetic nephropathy. Nat. Rev. Nephrol. 12, 13–26 (2016).
Molitch, M. E. et al. Diabetic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 87, 20–30 (2015).
United States Renal Data System. 2014 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. https://www.usrds.org/2014/view/ (2014).
Assogba, F. G. et al. Trends in the epidemiology and care of diabetes mellitus-related end-stage renal disease in France, 2007–2011. Diabetologia 57, 718–728 (2014).
Van Dijk, P. R. et al. Incidence of renal replacement therapy for diabetic nephropathy in the Netherlands: Dutch diabetes estimates (DUDE)-3. BMJ Open 5, e005624 (2015).
Toppe, C. et al. Renal replacement therapy due to type 1 diabetes; time trends during 1995–2010 — a Swedish population based register study. J. Diabetes Complicat. 28, 152–155 (2014).
Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 (1993).
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352, 837–853 (1998).
Gaede, P., Lund-Andersen, H., Parving, H.-H. & Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 358, 580–591 (2008).
Turner, M. W. The role of mannose-binding lectin in health and disease. Mol. Immunol. 40, 423–429 (2003).
Thiel, S. et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature 386, 506–510 (1997).
Law, S. K. A. & Reid, K. B. M. Complement 2nd edn (IRL Press at Oxford Univ. Press, 1995).
Mason, C. P. & Tarr, A. W. Human lectins and their role in viral infections. Molecules 20, 2229–2271 (2015).
Keshi, H. et al. Identification and characterization of a novel human collectin CL-K1. Microbiol. Immunol. 50, 1001–1013 (2006).
Nomura, A. et al. Role of complement in acute tubulointerstitial injury of rats with aminonucleoside nephrosis. Am. J. Pathol. 151, 539–547 (1997).
Nangaku, M. et al. Complement membrane attack complex (C5b-9) mediates interstitial disease in experimental nephrotic syndrome. J. Am. Soc. Nephrol. 10, 2323–2331 (1999).
Nangaku, M. et al. C6 mediates chronic progression of tubulointerstitial damage in rats with remnant kidneys. J. Am. Soc. Nephrol. 13, 928–936 (2002).
Morita, Y. et al. Complement activation products in the urine from proteinuric patients. J. Am. Soc. Nephrol. 11, 700–707 (2000).
Fortpied, J., Vertommen, D. & Van Schaftingen, E. Binding of mannose-binding lectin to fructosamines: a potential link between hyperglycaemia and complement activation in diabetes. Diabetes Metab. Res. Rev. 26, 254–260 (2010).
Fan, W. X. et al. Activation of the lectin complement pathway on human renal glomerular endothelial cells triggered by high glucose and mannose-binding lectin. Afr. J. Biotechnol. 10, 18539–18549 (2011).
Acosta, J. et al. Molecular basis for a link between complement and the vascular complications of diabetes. Proc. Natl Acad. Sci. USA 97, 5450–5455 (2000).
Qin, X. et al. Glycation inactivation of the complement regulatory protein CD59: a possible role in the pathogenesis of the vascular complications of human diabetes. Diabetes 53, 2653–2661 (2004).
Østergaard, J. et al. Mannose-binding lectin deficiency attenuates renal changes in a streptozotocin-induced model of type 1 diabetes in mice. Diabetologia 50, 1541–1549 (2007).
Østergaard, J. A. et al. Mannan-binding lectin in diabetic kidney disease: the impact of mouse genetics in a type 1 diabetes model. Exp. Diabetes Res. 2012, 678381 (2012).
Østergaard, J. A. et al. Diabetes-induced changes in mannan-binding lectin levels and complement activation in a mouse model of type 1 diabetes. Scand. J. Immunol. 77, 187–194 (2013).
Østergaard, J. A. et al. Increased autoreactivity of the complement-activating molecule mannan-binding lectin in a type 1 diabetes model. J. Diabetes Res. 2016, 1825738 (2016).
Hansen, T. K. et al. Elevated levels of mannan-binding lectin in patients with type 1 diabetes. J. Clin. Endocrinol. Metab. 88, 4857–4861 (2003).
Saraheimo, M. et al. Increased levels of mannan-binding lectin in type 1 diabetic patients with incipient and overt nephropathy. Diabetologia 48, 198–202 (2005).
Hansen, T. K. et al. Association between mannose-binding lectin and vascular complications in type 1 diabetes. Diabetes 53, 1570–1576 (2004).
Østergaard, J. A. et al. Increased all-cause mortality in patients with type 1 diabetes and high-expression mannan-binding lectin genotypes: a 12-year follow-up study. Diabetes Care 38, 1898–1903 (2015).
Kaunisto, M. A. et al. Elevated MBL concentrations are not an indication of association between the MBL2 gene and type 1 diabetes or diabetic nephropathy. Diabetes 58, 1710–1714 (2009).
Hovind, P. et al. Mannose-binding lectin as a predictor of microalbuminuria in type 1 diabetes: an inception cohort study. Diabetes 54, 1523–1527 (2005).
Hansen, T. K. et al. Association between mannose-binding lectin, high-sensitivity C-reactive protein and the progression of diabetic nephropathy in type 1 diabetes. Diabetologia 53, 1517–1524 (2010).
Zhao, S. Q. & Hu, Z. Mannose-binding lectin and diabetic nephropathy in type 1 diabetes. J. Clin. Lab. Anal. 30, 345–350 (2016).
Berger, S. P. et al. Low pretransplantation mannose-binding lectin levels predict superior patient and graft survival after simultaneous pancreas-kidney transplantation. J. Am. Soc. Nephrol. 18, 2416–2422 (2007).
Bijkerk, R. et al. Simultaneous pancreas-kidney transplantation in patients with type 1 diabetes reverses elevated MBL levels in association with MBL2 genotype and VEGF expression. Diabetologia 59, 853–858 (2016).
Hansen, T. K. et al. Mannose-binding lectin and mortality in type 2 diabetes. Arch. Intern. Med. 166, 2007–2013 (2006).
Elawa, G. et al. The predictive value of serum mannan-binding lectin levels for diabetic control and renal complications in type 2 diabetic patients. Saudi Med. J. 32, 784–790 (2011).
Zhang, N. et al. Association of levels of mannose-binding lectin and the MBL2 gene with type 2 diabetes and diabetic nephropathy. PLoS ONE 8, e83059 (2013).
Guan, L. Z., Tong, Q. & Xu, J. Elevated serum levels of mannose-binding lectin and diabetic nephropathy in type 2 diabetes. PLoS ONE 10, e0119699 (2015).
Holt, C. B. et al. Ficolin B in diabetic kidney disease in a mouse model of type 1 diabetes. Mediators Inflamm. 2015, 653260 (2015).
Østergaard, J. A. et al. Association of the pattern recognition molecule H-ficolin with incident microalbuminuria in an inception cohort of newly diagnosed type 1 diabetic patients: an 18 year follow-up study. Diabetologia 57, 2201–2207 (2014).
Jenny, L. et al. Plasma levels of mannan-binding lectin-associated serine proteases MASP-1 and MASP-2 are elevated in type 1 diabetes and correlate with glycaemic control. Clin. Exp. Immunol. 180, 227–232 (2015).
Mauer, S. M. et al. Pancreatic islet transplantation. Effects on the glomerular lesions of experimental diabetes in the rat. Diabetes 23, 748–753 (1974).
Mauer, S. M. et al. Studies of the rate of regression of the glomerular lesions in diabetic rats treated with pancreatic islet transplantation. Diabetes 24, 280–285 (1975).
Lee, C. S. et al. Renal transplantation in diabetes mellitus in rats. J. Exp. Med. 139, 793–800 (1974).
Xiao, X. et al. Cellular and humoral immune responses in the early stages of diabetic nephropathy in NOD mice. J. Autoimmun. 32, 85–93 (2009).
Yang, L. et al. Inflammatory gene expression in OVE26 diabetic kidney during the development of nephropathy. Nephron Exp. Nephrol. 119, e8–e20 (2011).
Wehner, H. et al. Glomerular changes in mice with spontaneous hereditary diabetes. Lab. Invest. 27, 331–340 (1972).
Kelly, K. J., Liu, Y., Zhang, J., Dominguez, J. H. Renal C3 complement component: feed forward to diabetic kidney disease. Am. J. Nephrol. 41, 48–56 (2015).
Woroniecka, K. I. et al. Transcriptome analysis of human diabetic kidney disease. Diabetes 60, 2354–2369 (2011).
Fujita, T. et al. Complement-mediated chronic inflammation is associated with diabetic microvascular complication. Diabetes Metab. Res. Rev. 29, 220–226 (2013).
Chiarelli, F., Verrotti, A., La Penna, G. & Morgese, G. Low serum C4 concentrations in type-1 diabetes mellitus. Eur. J. Pediatr. 147, 197–198 (1988).
Barnett, A. H. et al. Low plasma C4 concentrations: association with microangiopathy in insulin dependent diabetes. Br. Med. J. 289, 943–945 (1984).
Cooper, M. E. et al. Low serum C4 concentrations and microangiopathy in type I and type II diabetes. Br. Med. J. 292, 801 (1986).
Lhotta, K. et al. Complement C4 phenotypes in patients with end-stage renal disease. Nephron 72, 442–446 (1996).
Lhotta, K. et al. Polymorphism of complement C4 and susceptibility to IDDM and microvascular complications. Diabetes Care 19, 53–55 (1996).
Falk, R. J. et al. Neoantigen of the polymerized ninth component of complement. Characterization of a monoclonal antibody and immunohistochemical localization in renal disease. J. Clin. Invest. 72, 560–573 (1983).
Falk, R. J., Scheinman, J. I., Mauer, S. M. & Michael, A. F. Polyantigenic expansion of basement membrane constituents in diabetic nephropathy. Diabetes 32 (Suppl. 2), 34–39 (1983).
Falk, R. J. et al. Ultrastructural localization of the membrane attack complex of complement in human renal tissues. Am. J. Kidney Dis. 9, 121–128 (1987).
Uesugi, N. et al. Possible mechanism for medial smooth muscle cell injury in diabetic nephropathy: glycoxidationmediated local complement activation. Am. J. Kidney Dis. 44, 224–238 (2004).
Wada, T. & Nangaku, M. Novel roles of complement in renal diseases and their therapeutic consequences. Kidney Int. 84, 441–450 (2013).
Ghosh, P., Sahoo, R., Vaidya, A., Chorev, M. & Halperin, J. A. Role of complement and complement regulatory proteins in the complications of diabetes. Endocr. Rev. 36, 272–288 (2015).
Flyvbjerg, A. in Textbook of Diabetes 5th edn (eds Holt, R. I. G., Cockram, C., Flyvbjerg, A. & Goldstein, B. J.) 543–553 (Wiley-Blackwell, 2017).
Morgan, B. P. & Harris, C. L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 14, 857–877 (2015).
Wang, H. et al. The lectin-like domain of thrombomodulin ameliorates diabetic glomerulopathy via complement inhibition. Thromb. Haemost. 108, 1141–1153 (2012).
Li, L. et al. C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/β-catenin signaling pathway in diabetic kidney disease. Metabolism 64, 597–610 (2015).
Li, L. et al. C3a receptor antagonist ameliorates inflammatory and fibrotic signals in type 2 diabetic nephropathy by suppressing the activation of TGF-β/smad3 and IKBα pathway. PLoS ONE 9, e113639 (2014).
Fujita, T. et al. Complement activation accelerates glomerular injury in diabetic rats. Nephron 81, 208–214 (1999).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
PowerPoint slides
Glossary
- Polyol pathway
-
A pathway whereby aldoketo-reductase enzymes use nicotinic acid adenine dinucleotide phosphate (NADPH) to reduce sugar-derived carbonyl compounds to their respective sugar alcohols (polyols). Glucose is converted to sorbitol, and galactose to galactitol. Further, sorbitol is oxidized to fructose by sorbitol dehydrogenase, with a reduction of NAD+ to NADH. The rate-limiting step of the polyol pathway is regulated by aldose reductase.
- Hexosamine pathway
-
A pathway whereby glucosamine-6-phosphate is made from fructose-6-phosphate and an amino group from glutamine, and glucosamine-6-phosphate is acetylated through an exchange with acetyl-coenzyme A to form N-acetylglucosamine- 6-phosphate. The 6-phosphate forms N-acetyl-glucosamine-1-phosphate through the action of an isomerase. Finally, through a reaction with uridine triphosphate, uridine diphosphate-N-acetylglucosamine is formed.
Rights and permissions
About this article
Cite this article
Flyvbjerg, A. The role of the complement system in diabetic nephropathy. Nat Rev Nephrol 13, 311–318 (2017). https://doi.org/10.1038/nrneph.2017.31
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.31
This article is cited by
-
Large-scale causal analysis of gut microbiota and six common complications of diabetes: a mendelian randomization study
Diabetology & Metabolic Syndrome (2024)
-
Plasma proteome profiling reveals the therapeutic effects of the PPAR pan-agonist chiglitazar on insulin sensitivity, lipid metabolism, and inflammation in type 2 diabetes
Scientific Reports (2024)
-
Isoliquiritigenin attenuates high glucose-induced proliferation, inflammation, and extracellular matrix deposition in glomerular mesangial cells by suppressing JAK2/STAT3 pathway
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Glomerular proteomic profiling reveals early differences between preexisting and de novo type 2 diabetes in human renal allografts
BMC Nephrology (2023)
-
Systematic review of type 1 diabetes biomarkers reveals regulation in circulating proteins related to complement, lipid metabolism, and immune response
Clinical Proteomics (2023)