Abstract
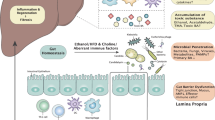
Hepatic steatosis is a multifactorial condition that is often observed in obese patients and is a prelude to non-alcoholic fatty liver disease. Here, we combine shotgun sequencing of fecal metagenomes with molecular phenomics (hepatic transcriptome and plasma and urine metabolomes) in two well-characterized cohorts of morbidly obese women recruited to the FLORINASH study. We reveal molecular networks linking the gut microbiome and the host phenome to hepatic steatosis. Patients with steatosis have low microbial gene richness and increased genetic potential for the processing of dietary lipids and endotoxin biosynthesis (notably from Proteobacteria), hepatic inflammation and dysregulation of aromatic and branched-chain amino acid metabolism. We demonstrated that fecal microbiota transplants and chronic treatment with phenylacetic acid, a microbial product of aromatic amino acid metabolism, successfully trigger steatosis and branched-chain amino acid metabolism. Molecular phenomic signatures were predictive (area under the curve = 87%) and consistent with the gut microbiome having an effect on the steatosis phenome (>75% shared variation) and, therefore, actionable via microbiome-based therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
09 August 2018
In the version of this article originally published, the received date was missing. It should have been listed as 2 January 2018. The error has been corrected in the HTML and PDF versions of this article.
References
Saltiel, A. R. & Kahn, C. R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 (2001).
Kahn, S. E., Hull, R. L. & Utzschneider, K. M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006).
Meex, R. C. R. & Watt, M. J. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 13, 509–520 (2017).
Petersen, M. C., Vatner, D. F. & Shulman, G. I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 13, 572–587 (2017).
Adams, L. A., Anstee, Q. M., Tilg, H. & Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 66, 1138–1153 (2017).
Dumas, M.-E. et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc. Natl Acad. Sci. USA 103, 12511–12516 (2006).
Le Roy, T. et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 62, 1787–1794 (2013).
Qin, N. et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64 (2014).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Turnbaugh, P. J. et al. A core gut microbiome in obese and lean twins. Nature 457, 480–484 (2009).
Ridaura, V. K. et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214 (2013).
Craciun, S. & Balskus, E. P. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc. Natl Acad. Sci. USA 109, 21307–21312 (2012).
Hoyles, L. et al. Metabolic retroconversion of trimethylamine N-oxide and the gut microbiota. Microbiome. 6, 73 (2018).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Dumas, M.-E. et al. Microbial–host co-metabolites are prodromal markers predicting phenotypic heterogeneity in behavior, obesity, and impaired glucose tolerance. Cell Rep. 20, 136–148 (2017).
Lonardo, A. et al. Fatty liver is associated with an increased risk of diabetes and cardiovascular disease—evidence from three different disease models: NAFLD, HCV and HIV. World J. Gastroenterol. 22, 9674–9693 (2016).
Houle, D., Govindaraju, D. R. & Omholt, S. Phenomics: the next challenge. Nat. Rev. Genet. 11, 855–866 (2010).
Dumas, M.-E., Kinross, J. & Nicholson, J. K. Metabolic phenotyping and systems biology approaches to understanding metabolic syndrome and fatty liver disease. Gastroenterology 146, 46–62 (2014).
Le Chatelier, E. et al. Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546 (2013).
Newgard, C. B. et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326 (2009).
Jang, C. et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 22, 421–426 (2016).
Karlsson, F. H. et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498, 99–103 (2013).
Forslund, K. et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528, 262–266 (2015).
Wu, H. et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 23, 850–858 (2017).
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 59, 1121–1140 (2016).
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402 (2016).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Pedersen, H. K. et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535, 376–381 (2016).
Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012).
Holmes, E. et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 453, 396–400 (2008).
Peng, Y., Leung, H. C. M., Yiu, S. M. & Chin, F. Y. L. IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 28, 1420–1428 (2012).
Besemer, J. Heuristic approach to deriving models for gene finding. Nucleic Acids Res. 27, 3911–3920 (1999).
Zhu, W., Lomsadze, A. & Borodovsky, M. Ab initio gene identification in metagenomic sequences. Nucleic Acids Res. 38, e132 (2010).
Li, J. et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 32, 834–841 (2014).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Shoaie, S. et al. Quantifying diet-induced metabolic changes of the human gut microbiome. Cell Metab. 22, 320–331 (2015).
Spencer, M. D. et al. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 140, 976–986 (2011).
Pallister, T. et al. Hippurate as a metabolomic marker of gut microbiome diversity: modulation by diet and relationship to metabolic syndrome. Sci. Rep. 7, 13670 (2017).
Kadar, H. et al. A multiplexed targeted assay for high-throughput quantitative analysis of serum methylamines by ultra performance liquid chromatography coupled to high resolution mass spectrometry. Arch. Biochem. Biophys. 597, 12–20 (2016).
Plovier, H. et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23, 107–113 (2017).
Schugar, R. C. et al. The TMAO-producing enzyme flavin-containing monooxygenase 3 regulates obesity and the beiging of white adipose tissue. Cell Rep. 19, 2451–2461 (2017).
Tarca, A. L. et al. A novel signaling pathway impact analysis. Bioinformatics 25, 75–82 (2009).
Davidovic, L. et al. A metabolomic and systems biology perspective on the brain of the fragile X syndrome mouse model. Genome Res. 21, 2190–2202 (2011).
Rodriguez-Martinez, A. et al. MetaboSignal: a network-based approach for topological analysis of metabotype regulation via metabolic and signaling pathways. Bioinformatics 33, 773–775 (2017).
Biddinger, S. B. et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat. Med. 14, 778–782 (2008).
Michael, M. D. et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 6, 87–97 (2000).
Fujisaka, S. et al. Antibiotic effects on gut microbiota and metabolism are host dependent. J. Clin. Invest. 126, 4430–4443 (2016).
Nicolas, S. et al. Transfer of dysbiotic gut microbiota has beneficial effects on host liver metabolism. Mol. Syst. Biol. 13, 921 (2017).
Grasset, E. et al. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric NO-dependent and gut–brain axis mechanism. Cell Metab. 25, 1075–1090.e5 (2017).
Latorre, J. et al. Decreased lipid metabolism but increased FA biosynthesis are coupled with changes in liver microRNAs in obese subjects with NAFLD. Int. J. Obes. (Lond.) 41, 620–630 (2017).
Cotillard, A. et al. Dietary intervention impact on gut microbial gene richness. Nature 500, 585–588 (2013).
Sharifnia, T. et al. Hepatic TLR4 signaling in obese NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G270–G278 (2015).
Thaiss, C. A. et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 540, 540–551 (2016).
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015).
Boursier, J. et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 63, 764–775 (2016).
Loomba, R. et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 25, 1054–1062.e5 (2017).
Serino, M. et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 61, 543–553 (2012).
Claesson, M. J. et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184 (2012).
Falony, G. et al. Population-level analysis of gut microbiome variation. Science 352, 560–564 (2016).
Grasa, M. D. M. et al. Modulation of SHBG binding to testosterone and estradiol by sex and morbid obesity. Eur. J. Endocrinol. 176, 393–404 (2017).
American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care 41, S13–S27 (2018).
Dieterle, F., Ross, A., Schlotterbeck, G. & Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 78, 4281–4290 (2006).
Fleige, S. & Pfaffl, M. W. RNA integrity and the effect on the real-time qRT–PCR performance. Mol. Aspects Med. 27, 126–139 (2006).
Smyth, G. K. "limma: Linear Models for Microarray Data" in Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Series in Statistics for Biology and Health (eds Gentleman, R., Carey, V., Huber, W., Irizarry, R., Dudoit, S.) 397–420 (Springer, New York, NY, 2005).
Zhang, J. D. & Wiemann, S. KEGGgraph: a graph approach to KEGG PATHWAY in R and bioconductor. Bioinformatics 25, 1470–1471 (2009).
Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128 (2013).
Kuleshov, M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97 (2016).
Lluch, J. et al. The characterization of novel tissue microbiota using an optimized 16S metagenomic sequencing pipeline. PLoS ONE 10, e0142334 (2015).
Escudié, F. et al. FROGS: find, rapidly, OTUs with galaxy solution. Bioinformatics 34, 1287–1294 (2018).
Segata, N. et al. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat. Methods 9, 811–814 (2012).
Truong, D. T. et al. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat. Methods 12, 902–903 (2015).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Dumas, M.-E. et al. Assessment of analytical reproducibility of 1H NMR spectroscopy based metabonomics for large-scale epidemiological research: the INTERMAP Study. Anal. Chem. 78, 2199–2208 (2006).
Smilde, A. K., Kiers, H. A. L., Bijlsma, S., Rubingh, C. M. & van Erk, M. J. Matrix correlations for high-dimensional data: the modified RV-coefficient. Bioinformatics 25, 401–405 (2009).
Cloarec, O. et al. Evaluation of the orthogonal projection on latent structure model limitations caused by chemical shift variability and improved visualization of biomarker changes in 1H NMR spectroscopic metabonomic studies. Anal. Chem. 77, 517–526 (2005).
Blaise, B. J. et al. Metabotyping of Caenorhabditis elegans reveals latent phenotypes. Proc. Natl Acad. Sci. USA 104, 19808–19812 (2007).
Acknowledgements
We thank N. J. Gooderham for critical reading of the manuscript. This work was supported by EU-FP7 FLORINASH (Health-F2-2009-241913) to R.B., M.F., J.-M.F.-R., F.F., C.P., E.H. and J.K.N. This work used the computing resources of the UK MEDical BIOinformatics partnership—aggregation, integration, visualization and analysis of large, complex data (UK MED-BIO), which is supported by the Medical Research Council (grant number MR/L01632X/1). L.H. is in receipt of an MRC Intermediate Research Fellowship in Data Science (MR/L01632X/1, UK MED-BIO). This work was also supported by funding to R.B. ((Région Midi-Pyrénées 2009-2014 RPV09003BBA), Agence Nationale de la Recherche ANR 09-GEN TRANSFLORAP and GAD 08-2_378258), to M.F. (Ministry of University (MIUR) Progetti di Ricerca di Interesse Nazionale (PRIN) protocol number 2015MPESJS_004, Ministry of Health Ricerca Finalizzata RF-2011-02349921, Fondazione Roma call for Non-Communicable Diseases NCD 2014), to J.-M.F.-R. (Ministry of Health FIS project 15/01934, CIBERobn Pathophysiology of Obesity and Nutrition and FEDER funds) and to M.-E.D. (EU METACARDIS under agreement HEALTH-F4-2012-305312, Neuron II under agreement 291840 and the MRC MR/M501797/1). We acknowledge the support of the Imperial College National Institute of Biomedical Research andthe Clinical Phenotyping Centre for support.
Author information
Authors and Affiliations
Contributions
R.B., J.-M.F.-R., M.F., F.L., F.F., C.P., E.H. and J.K.N. designed the study and supervised all parts of the project. R.B. is the project leader and chaired the consortium. M.-E.D. led data integration and elaborated the primary interpretation of analytical outcomes with L.H., in close collaboration with M.F., J.-M.F.-R. and R.B. L.H. implemented the microarray data analysis workflow. C.T. and M.W. developed the data repository. J.A. developed the metagenomic data analysis pipeline in collaboration with L.H. S.A.B. supervised the development of the data repository and the pipeline. L.H., J.A., R.H.B. and M.-E.D. performed the data analyses. J.-M.F.-R. and M.F. designed the clinical protocol and oversaw the clinical activities. M.C., F.D., I.C., O.P., P.G., J.P., G.X. and W.R. recruited and phenotyped patients and collected biological samples and physiological data. M.S., V.A. and V.B.-B. performed the RNA and DNA extractions. R.B. and M.S. supervised the DNA sequencing and gene profiling. J.L.L. and J.M.M.-N. performed the cell culture experiments. F.B., E.A., J.Charpentier and C.H. performed the animal work. R.H.B., J.Chilloux and L.M.-G. performed the metabolic profiling of plasma and urine by 1H-NMR. E.H. and J.K.N. supervised the metabolic profiling. A.M. performed the methylamine quantifications. L.H. and M.-E.D. drafted the first versions of the paper with critical and substantial contributions from M.F., J.-M.F.-R. and R.B. All authors provided support and constructive criticism throughout the project and approved the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
L.H., J.-M.F.-R., M.F., R.H.B., J.L.L., E.H., J.K.N., C.P., R.B. and M.-E.D. are named as co-inventors on pending patents held by INSERM Transfert, INSERM, University of Rome Tor Vergata, University of Girona and Imperial College on NAFLD diagnostics and have the right to receive royalty payments for inventions or discoveries related to NAFLD diagnostics.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12
Supplementary Tables
Supplementary Tables 1–13
Rights and permissions
About this article
Cite this article
Hoyles, L., Fernández-Real, JM., Federici, M. et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med 24, 1070–1080 (2018). https://doi.org/10.1038/s41591-018-0061-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0061-3
This article is cited by
-
Development of non-alcoholic steatohepatitis is associated with gut microbiota but not with oxysterol enzymes CH25H, EBI2, or CYP7B1 in mice
BMC Microbiology (2024)
-
Correlation of non-alcoholic fatty liver disease and gut microflora: clinical reports and treatment options
Egyptian Liver Journal (2024)
-
Ganoderma lucidum polysaccharide ameliorates cholesterol gallstone formation by modulating cholesterol and bile acid metabolism in an FXR-dependent manner
Chinese Medicine (2024)
-
A nanoemulsion targeting adipose hypertrophy and hyperplasia shows anti-obesity efficiency in female mice
Nature Communications (2024)
-
Multi-kingdom microbial signatures in excess body weight colorectal cancer based on global metagenomic analysis
Communications Biology (2024)