Abstract
There is a need for treatments that can safely promote regulatory lymphocyte responses. T cells express GABA receptors (GABAA-Rs) and GABA administration can inhibit Th1-mediated processes such as type 1 diabetes and rheumatoid arthritis in mouse models. Whether GABAA-R agonists can also inhibit Th17-driven processes such as experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS), is an open question. GABA does not pass through the blood-brain barrier (BBB) making it ill-suited to inhibit the spreading of autoreactivity within the CNS. Homotaurine is a BBB-permeable amino acid that antagonizes amyloid fibril formation and was found to be safe but ineffective in long-term Alzheimer’s disease clinical trials. Homotaurine also acts as GABAA-R agonist with better pharmacokinetics than that of GABA. Working with both monophasic and relapsing-remitting mouse models of EAE, we show that oral administration of homotaurine can (1) enhance CD8+CD122+PD-1+ and CD4+Foxp3+ Treg, but not Breg, responses, (2) inhibit autoreactive Th17 and Th1 responses, and (3) effectively ameliorate ongoing disease. These observations demonstrate the potential of BBB-permeable GABAA-R agonists as a new class of treatment to enhance CD8+ and CD4+ Treg responses and limit Th17 and Th1-medaited inflammation in the CNS.
Similar content being viewed by others
Introduction
There is an unmet need for clinical treatments that can safely limit the progression of chronic inflammatory disorders. This is especially true for T-cell mediated autoimmune disorders such as type 1 diabetes (T1D), rheumatoid arthritis (RA), and multiple sclerosis (MS) in which inhibiting established autoimmune responses against the target tissue may require life-time treatment.
Rodent and human T cells express receptors for the nonprotein amino acid γ-aminobutyric acid (GABA)1,2,3,4,5. GABA is a commonly used neurotransmitter in the CNS and its neurobiological activities in brain development and function have been extensively studied. The immunobiological activities of GABA, however, are just beginning to be elucidated. There are two types of GABA-receptors (GABA-Rs) that are encoded by distinct gene families and their activation induces different pathways; GABAA-Rs are fast-acting chloride channels and GABAB-Rs are slow-acting G-protein coupled receptors6,7. Rodent and human T cells expresses functional GABAA-Rs but appear unresponsive to GABAB-R-specific agonists1,3,4,5. In mice, GABA administration limited delayed type hypersensitivity (DTH) responses and inhibited or reversed disease in models of T1D1,2,5,8,9,10, RA11, and limited inflammation and disease in type 2 diabetes models12,13,14. Studies of the mechanisms underlying those observations revealed that GABA treatment inhibited the development of autoreactive Th1 responses while also increasing CD4+ Tregs. These preclinical studies provided the basis for an ongoing clinical trial in which GABA is being given to individuals newly diagnosed with T1D (NCT02002130).
Whether this therapeutic approach can be extended to EAE and potentially to MS is unclear. First, while autoreactive Th1 cells are thought to be primary drivers of DTH responses, T1D, and RA disease progression, autoreactive Th17 responses are thought to play a major pathogenic role in EAE and MS15,16. Second, while DTH, T1D, and RA are mediated by peripheral autoimmune T cell responses, disease relapses in EAE and MS are thought be due to the spreading of T cell autoreactivity within the CNS17,18,19. Orally administered GABA, however, has little to no ability to pass through the BBB20, making it ill-suited to inhibit the spreading of T cell autoreactivities within the CNS. To circumvent that limitation, Steinman and colleagues21 showed that two clinically applicable BBB-permeable anti-seizure medications, topiramate and vigabatrin, that increase GABAergic tone, inhibited EAE. Topiramate, however, primarily affects other ion channels (e.g., sodium and calcium channels) and enzymes22,23 and vigabatrin does not bind to GABA-Rs but rather inhibits GABA transaminase23, and both drugs have multiple adverse effects. Other studies have shown that the anti-alcohol dependence drug acamprosate, which has structural similarities with GABA, can limit EAE24, but recent studies indicate that acamprosate does not act directly through GABAA-Rs25,26. Benzodiazepines and barbiturates are BBB-permeable GABAA-R positive allosteric modulators that can potentiate the opening of GABAA-R chloride channels, but only after a GABAA-R agonist opens the channel, and these drugs can be addictive. Accordingly, there is a need for BBB-permeable GABAA-R-specific agonists with excellent safety profiles that could potentially provide a new class of drugs for limiting inflammation in the CNS.
Homotaurine is a natural amino acid found in algae. Homotaurine emerged as a leading candidate from a screen for compounds that physically interfered with the ability of amyloid peptide to form fibrils in vitro27,28. Subsequent studies found that oral homotaurine can pass through the BBB and limit amyloid plaque deposition in the brain of transgenic mice that over-expressed human amyloid protein27,28. Based on these observations, homotaurine (also known as Tramiprosate or Alzhemed) was tested in a large double-blind randomized phase III clinical trial for its ability to slow cognitive loss over 1.5 years in patients with Alzheimer’s disease29,30. While homotaurine treatment was found to not slow cognitive decline, it had an excellent safety profile in this long-term study. Recently, it has become appreciated that homotaurine can also act as a GABAA-R-specific agonist. Homotaurine has >3-fold higher affinity for classical GABAA-Rs and a longer half-life than GABA in plasma (≈3 hours vs. 20 minutes for GABA after intravenous or intraperitoneal injection)28,31,32,33,34. Homotaurine’s pharmacokinetic properties, along with its safety record in humans, make it an appealing candidate for treating inflammation in the CNS. We therefore tested whether homotaurine could be repurposed for treating murine EAE. After finding homotaurine was effective in two different murine EAE models, we studied the treatment’s potential therapeutic mechanisms and observed, for the first time, that in addition to promoting CD4+Foxp3+ Treg responses, GABAA-R activation also enhances CD8+CD122+PD-1+ Treg responses and inhibits Th17 responses. Thus, GABAA-Rs are a new druggable target for enhancing CD8+ Treg responses and inhibiting Th17 responses in inflammation-related disorders.
Results and Discussion
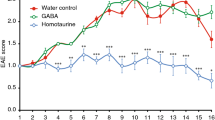
We first tested the ability of homotaurine to limit disease progression in the chronic model of EAE that was induced by immunizing C57BL/6 mice with a myelin oligodendrocyte glycoprotein peptide (MOG35–55)35. Eleven to thirteen days after receiving the encephalitic regimen, all of the mice began to develop EAE. When an animal reached an EAE severity score of 1 it was randomly assigned to receive plain drinking water, or water containing homotaurine (0.25 mg/ml) for the rest of the observation period. This homotaurine dose was chosen based on our dosing studies of homotaurine’s ability to reverse disease in newly diabetic NOD mice (manuscript in preparation). Control and homotaurine-treated groups of mice drank similar amounts of water each day (≈4 ml/mouse/day). While the control group of mice quickly progressed to more severe EAE, the homotaurine treatment group of mice remained at low clinical score of EAE (Fig. 1A).
Homotaurine inhibits EAE progression after the onset of symptoms in both monophasic and relapsing-remitting mouse models of MS. (A) C57BL/6 mice were immunized with MOG35–55 and monitored daily for clinical signs of EAE as described in Methods. Eleven to thirteen days post-immunization all of the mice developed EAE. When the mice reached an EAE score of 1 they were randomized to receive plain water or water containing homotaurine (0.25 mg/ml). Graph shows mean EAE scores ± SEM for mice that received plain water (circles) and homotaurine (triangles) after EAE onset. N = 5 mice/group, overall p = 0.01, by Kruskal-Wallis analysis. (B) SJL mice were immunized with PLP139–151 and ten to twelve days later all of the mice develeoped clinical symptoms. When the mice reached an EAE score of 1 they were randomized to receive plain water or water containing homotaurine. Graph shows mean EAE scores ± SEM for mice that received plain water (circles) or homotaurine (triangles) continuously after EAE onset. N = 8 mice/group, *p < 0.05, overall p < 0.01. (C) Spinal cord (left panel) and cerebellum (right panel) in control and homotaurine-treated PLP139–151 immunized SJL mice. Twenty-seven days after treatment, adjacent sections were stained with hematoxylin and eosin (H &E, top row) and luxol fast blue or black gold (bottom row). Arrow in H&E stained sections indicates infiltrates. Inserts in black gold stained cerebellum images show boxed areas magnified 1.5X revealing greater myelination and fine myelin fibers in homotaurine-treated mice (arrows). Left panel scale bar = 1 mm, right panel scale bar = 250 µm.
We next tested homotaurine treatment in a relapsing-remitting model of EAE that was induced by immunizing SJL mice with a proteolipid protein peptide (PLP139–151)36. Ten to 12 days after receiving the encephalitic regimen, all of the mice began to develop EAE. When an animal reached an EAE severity score of 1 it was randomly assigned to receive plain drinking water, or water containing homotaurine (0.25 mg/ml) for the rest of the study. Control mice displayed classical relapsing-remitting EAE. Homotaurine-treated mice exhibited a reduced mean EAE severity throughout the observation period compared to those given plain water, and eventually displayed almost complete remission (Fig. 1B). Histological analysis of their brains and spinal cords revealed reduced mononuclear cell infiltration and areas of myelin loss in the cerebellum and spinal cords of homotaurine-treated mice (Fig. 1C). Thus, treatment with homotaurine after the clinical onset of EAE inhibited disease progression in both monophasic and relapsing-remitting mouse models of MS. Previous studies using GABA to inhibit T1D and RA in mouse models could not distinguish the contributions of GABAA-Rs versus GABAB-Rs to those observations. Our results using homotaurine point directly to GABAA-R-mediated pathways as major components of GABA’s anti-inflammatory effects.
To begin to understand the basis for homotaurine’s therapeutic effects, we next examined homotaurine’s effects on lymphocytes since T cells are the major mediators of EAE and MS. SJL mice were immunized with PLP139–151 and 10 days later (when most mice had an EAE score of about 1), the mice were given plain water or water containing homotaurine (0.25 mg.ml) continuously for five days after which their splenic T cells were analyzed by ELISPOT. Consistent with previous studies of GABA treatment2,5,8,10,11, we observed that homotaurine treatment significantly decreased the frequency of splenic IFNγ (Th1) responses, but increased IL-10-secreting responses to PLP139–151 (Fig. 2). In addition, we show for the first time, that GABAA-R activation significantly reduces the frequency of PLP139–151-reactive IL-17A (Th17) spot-forming colonies (SFC) (Fig. 2). These IL-17A SFC represent antigen-specific activated/memory CD4+ T cells since peptide 13mers do not stimulate CD8+ T cells effectively, and it has been demonstrated in the PLP139–151/SJL model that IL-17 secreting SFC arise from CD4+ cells and not other cell types37. Homotaurine had no effect on Th2 frequencies (data not shown).
Homotaurine treatment at time of clinical EAE onset reduces the frequency of autoreactive IL-17A- and IFNγ-secreting T cell responses, while increasing IL-10-secreting responses. Mice were PLP139–151 immunized and after reaching an EAE score of 1, they received plain water or homotaurine for five days. Splenic T cells were isolated from individual mice and the percentages of IL-17A, IFNγ and IL-10 secreting T cells responding to PLP139–151 were measured by ELISPOT. Data shown are the mean SFC ± SEM of each group of mice (n = 5 per group) from two separate experiments. IL-4 responses to PLP139–151 were at background levels (data not shown). Wells containing cells from control or homotaurine-treated mice that were incubated with medium alone had 0–5 SFC. Pairwise comparisons were performed by 2-tailed Student’s t test. **p < 0.01 vs. the controls.
GABA treatment can promote CD4+ Treg responses in mice8,12. It is unknown whether GABAA-R activation might also modulate CD8+CD122+PD-1+ and CD8+CD122+PD-1− regulatory T cells or IL-10-secreting Bregs which can play crucial roles in inhibiting inflammation and T cell autoimmunity. We found that the percentages of splenic CD4+Foxp3+ Tregs (Fig. 3A) and CD8+CD122+PD-1+, but not CD8+CD122+PD-1−, Tregs (Fig. 3B,C) in the homotaurine-treated mice were significantly higher than in the controls. There was no significant difference in the frequency of splenic CD19+IL-10+ Bregs (data not shown).
Homotaurine treatment enhances CD4+ and CD8+ Treg responses in mice. SJL mice were immunized with PLP139–151 in 50% CFA and when the mice developed EAE with a score of 1, they were randomized and provided with plain water and water containing 0.25 mg/ml homotaurine for five days. The percentages of splenic (A) CD4+Foxp3+, (B,C) CD8α+CD122+ and CD8α+CD122+PD-1+ Tregs were determined by flow cytometry. The cells were gated first on living lymphocytes and CD4+ or CD8+ and the percentages of CD4+Foxp3+, CD8α+CD122+PD-1− and CD8α+CD122+PD-1+ Tregs were analyzed. Data are representative flow cytometry charts or expressed as the mean ± SEM of each group of mice (n = 5 per group) from two separate experiments. *p < 0.05, **p < 0.01 vs. the controls. (D) Splenic CD8+CD122+PD-1+ and CD8+CD122+PD-1− Tregs were sorted by flow cytometry and mixed with lymph node mononuclear cells from PLP139–151-immunized SJL mice at the indicated ratios and assessed for PLP139–151-stimulated T cell proliferation. Data are expressed as mean ± SD of each group of cells. The cultures with responders in medium alone served the negative controls (with CPM of 850–940) and peptide-stimulated cultures without any inhibitor provided positive controls. Additional positive control cells were stimulated with PPD or anti-CD3 (with CPM of 20821–26759). *p < 0.05, **p < 0.01 vs. the peptide-stimulated positive controls.
To test the inhibitory function of CD8+CD122+PD-1+ and CD8+CD122+PD-1− Tregs, SJL mice were immunized with PLP139–151 and 10 days later, they were randomized to receive homotaurine (0.25 mg/ml) through their drinking water, or plain water for the next six days. Their splenic CD8+CD122+PD-1+ and CD8+CD122+PD-1− Tregs were sorted by flow cytometry and mixed with lymph node mononuclear cells from PLP139–151-immunized SJL mice for proliferation assays. Co-culture with CD8+CD122+PD-1+ Tregs, but not CD8+CD122+PD-1− Tregs, from either homotaurine-treated or control (plain water)-treated mice significantly inhibited the proliferation of responders in a dose-dependent manner (Fig. 3D). The inhibitory effect of CD8+CD122+PD-1+ cells from homotaurine-treated mice was comparable to those from control mice, indicating that homotaurine treatment did not significantly alter their suppressive activity. These data, together with the increased frequency of CD4+Foxp3+ and CD8+CD122+PD-1+ Tregs in the homotaurine-treated mice indicate that CD4+Foxp3+ and CD8+CD122+PD-1+ Tregs play an important role in inhibiting the pathogenic T cell autoimmunity, contributing to the decreased severity of EAE.
Very few of the many drugs that can inhibit EAE successfully translated to the clinic. Factors that contribute to this failure include; (1) inherent differences between EAE and MS, (2) toxicity or side-effects in humans, and (3) supra-physiological doses were used in the EAE studies. In regard to those issues, human T cells express GABAA-Rs whose activation can limit PBMC inflammatory responses3,4,5,38, homotaurine appears to be safe for long-term human use, and using FDA guidelines (www.fda.gov/downloads/Drugs/…/Guidances/UCM078932.pdf) for scaling mouse doses to the equivalent human dosage, the homotaurine dose we used to inhibit EAE was 6–10-fold lower than that used in the Alzheimer’s disease clinical trials.
Murine and human T cells express relatively high levels of transcripts encoding the δ and ρ2 subunits of GABAA-Rs2,3,4,5,38. The δ and ρ2 subunits are of particular interest because thay are found in “extrasynaptic” GABAA-Rs that can be orders of magnitude more sensitive to GABA than the typical GABAA-Rs subtypes that are clustered in neuronal synapses39,40,41,42,43,44. These δ and /or ρ2 subunits may confer T cells with sensitivity to low levels of GABA and contribute to CNS “immunological privilege”. In this scenario, endogenous CNS GABA may help regulate the low-frequency CNS-reactive T cells that spontaneously activate under normal conditions, but it is insufficient to regulate the large number of autoreactive T cells that are experimentally activated in EAE. As we show here, administration of a BBB-permeable high-affinity GABAA-R agonist is capable of limiting CNS autoreactivity under those conditions. Additionally, homotaurine may have acted on antigen presenting cells (APC) such as macrophages, dendritic cells, and microglia that contribute to EAE pathogenesis21,45,46. These APC also express GABAA-Rs and GABAA-R agonists have been shown to inhibit their inflammatory activity11,21,47,48,49.
In summary, our findings provide insights into the actions of GABAA-Rs on lymphocytes, as well as a proof-of-principle for a potential new class of drug treatment for enhancing Treg responses and limiting autoreactive Th17 cells. Our findings have potential for treating MS and may have broader applications for ameliorating other inflammation-related disorders of the CNS.
Methods
EAE induction and treatment
All experimental protocols were approved by the UCLA Animal Protection Committee and carried out in accordance with relevant guidelines. Nine to ten weeks old female C57BL/6 or SJL mice were obtained from the Jackson Laboratory and housed in a specific pathogene-free facility with free access of food and water. C57BL/6 mice were immunized subcutaneously with MOG35–55 (200 µg) in 50% IFA containing Mycobacterium tuberculosis H37R (5 mg/ml, Difco) in multiple sites near the base of their tail on day 1 and injected intraperitoneally with pertussis toxin (200 ng/mouse) on day 0 and 2. Individual SJL mice were immunized with PLP139–151 (100 µg/mouse), as described above, but without pertussis toxin injection. The mice were monitored for EAE onset daily: 0, no disease; 1, limp tail; 2, hind limb weakness; 3, complete hind limb paralysis; 4, quadriplegia; and 5, death. Mice that were in between the clear-cut gradations were scored intermediate in increments of 0.5. When the mice developed EAE with a score of 1 at 10–12 days post-immunization, they were randomized to receive plain water or water containing homotaurine (0.25 mg/ml (Sigma-Aldrich A76109), an optimal dose based on preliminary studies of T1D prevention in NOD mice).
Histology
Mice were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer, fixed overnight at 4 °C, cryoprotected and frozen. The brain and spinal cord cryostat sections (10 and 20 μm) were directly mounted on slides or collected into PBS with 0.06% NaN3. The sections were mounted onto Superfrost + slides and stained with H&E or Black Gold II. Some mouse brain and spinal cord tissues were paraffin-embedded after fixation and stained with H&E or luxol fast blue. The sections were imaged under a light microscope.
ELISPOT assays for IFN, IL-17A, IL-10
SJL mice were immunized with 100 µg PLP139–151 in 50% IFA containing 5 mg/ml Mycobacterium tuberculosis H37R. When the mice developed EAE with a score of 1, the mice were randomized and received plain water or water containing 0.25 mg/ml homotaurine for 5 days. Their splenic mononuclear cells were isolated and the frequency of IFNγ, IL-17A, IL-4 and IL-10-secreting T cells responding to antigens were determined by ELISPOT as described previously10 with the addition that rat anti-mouse interleukin IL-17 (4 μg/ml, clone TC-11-18H10, BD Biosciences) and biotin-conjugated anti-IL-17 (5.0 μg/ml; Pharmingen, clone TC-11-8H4.1) were used as capture and detection antibodies for IL-17A, respectively. Briefly, splenic mononuclear cells (106/well) were stimulated in duplicate with PLP139-151 (15 µg/ml), positive control PPD (5 µg/ml) or mouse serum albumin (50 µg/ml, Sigma) in HL-1 medium for 24 or 48 h. The spot forming colonies (SFC) in each well were counted in a blinded manner.
FACS
When PLP139–151 immunized mice developed EAE with a score of 1, they were randomized to receive plain water or water containing 0.25 mg/ml homotaurine for 5 days. Splenic mononuclear cells (106/tube) from the control and homotaurine-treated groups were treated with 1 µg anti-CD16/anti-CD32 and stained with FITC-anti-CD8α, APC-anti-CD122 and PE-anti-PD-1. After being washed, the frequency of CD8+CD122+PD1− and CD8+CD122+PD-1+ regulatory T cells was determined by flow cytometry. In addition, splenic mononuclear cells (106/tube) were stained with FITC-anti-CD4, fixed, permeabilized and intracellularly stained with PE-anti-Foxp3. The frequency of CD4+Foxp3+ Tregs was determined by flow cytometry. Moreover, splenic mononuclear cells (106/tube) were stimulated with 50 nM PMA and 1 µM ionomycine (Sigma) for 2 h and in the presence of monensin for another 3 h. The cells were stained with FITC-anti-CD19, fixed, permeabilized and intracellularly stained with PE-anti-IL-10. The cells with isotype controls served as the negative controls. The frequency of CD19+IL-10+ Bregs was determined by flow cytometry.
Functional assessment of CD8+ Treg suppressor activity
Female SJL mice were injected with 200 µg PLP139–51 in 50% CFA and nine days later, the mice were randomized and provided with, or without, homotaurine in their drinking water (0.25 mg/ml) for 6 days. The mice were then sacrificed, their splenic mononuclear cells were isolated, and CD8+ T cells were enriched by negative selection using the Majosort mouse CD8 T cell isolation kit (BioLegend, San Diego). The isolated CD8+ T cells were stained with FITC-anti-CD8, APC-anti-CD122 and PE-anti-PD-1 (BioLegend). The CD8+CD122+PD-1+ and CD8+CD122+PD-1− Tregs were sorted by flow cytometry and used as inhibitors. In addition, SJL mice were immunized with 100 µg PLP139–151 in 50% CFA in their footpads and nine days later, their draining lymph node mononuclear cells were isolated and used as responders. To test the inhibitory function of CD8+ Tregs, lymph node mononuclear cells (1 × 105 cells/well) were mixed in triplicate with CD8+CD12+PD-1+ or CD8+CD122+PD-1− Tregs from either control or homotaurine-treated mice at ratios of 0.2, 0.1 or 0.05 (inhibitors to responders) and stimulated with PLP139–151 (20 µg/ml) for 96 hours. Some responders received medium alone (negative control) and other responders were stimulated with peptide antigen in the absence of inhibitory cells (positive control). Moreover, some responders were stimulated with PPD (10 µg/ml) or anti-CD3 (1 µg/ml) and served as additional positive controls. During the last 16-hour culture, individual wells of cells were exposed to 1 µci 3H-thymidine and the 3H-thymidine uptakes were measured by a β-counter.
Statistics
EAE scores were evaluated using Kruskal-Wallis test. Pairwise comparisons were performed by 2-tailed Student’s t test. P < 0.05 was considered statistically significant.
References
Tian, J., Chau, C., Hales, T. G. & Kaufman, D. L. GABA(A) receptors mediate inhibition of T cell responses. J Neuroimmunol 96, 21–28 (1999).
Tian, J. et al. Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J Immunol 173, 5298–5304 (2004).
Alam, S., Laughton, D. L., Walding, A. & Wolstenholme, A. J. Human peripheral blood mononuclear cells express GABAA receptor subunits. Mol Immunol 43, 1432–1442 (2006).
Mendu, S. K., Bhandage, A., Jin, Z. & Birnir, B. Different subtypes of GABA-A receptors are expressed in human, mouse and rat T lymphocytes. PLoS One 7, e42959 (2012).
Prud’homme, G. J. et al. GABA Protects Human Islet Cells Against the Deleterious Effects of Immunosuppressive Drugs and Exerts Immunoinhibitory Effects Alone. Transplantation 96, 616–623, https://doi.org/10.1097/TP.0b013e31829c24be (2013).
Olsen, R. W. & Tobin, A. J. Molecular biology of GABAA receptors. Faseb J 4, 1469–1480 (1990).
Bettler, B., Kaupmann, K., Mosbacher, J. & Gassmann, M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev 84, 835–867 (2004).
Soltani, N. et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci USA 108, 11692–11697, https://doi.org/10.1073/pnas.1102715108 (2011).
Mendu, S. K. et al. Increased GABA(A) channel subunits expression in CD8(+) but not in CD4(+) T cells in BB rats developing diabetes compared to their congenic littermates. Mol Immunol 48, 399–407, https://doi.org/10.1016/j.molimm.2010.08.005 (2011).
Tian, J., Dang, H., Nguyen, A. V., Chen, Z. & Kaufman, D. L. Combined therapy with GABA and proinsulin/alum acts synergistically to restore long-term normoglycemia by modulating T-cell autoimmunity and promoting beta-cell replication in newly diabetic NOD mice. Diabetes 63, 3128–3134, https://doi.org/10.2337/db13-1385 (2014).
Tian, J., Yong, J., Dang, H. & Kaufman, D. L. Oral GABA treatment downregulates inflammatory responses in a mouse model of rheumatoid arthritis. Autoimmunity 44, 465–470 (2011).
Tian, J. et al. Oral treatment with gamma-aminobutyric acid improves glucose tolerance and insulin sensitivity by inhibiting inflammation in high fat diet-fed mice. PLoS One 6, e25338, https://doi.org/10.1371/journal.pone.0025338 (2011).
Sohrabipour, S., Reza Sharifi, M., Talebi, A., Sharifi, M. & Soltani, N. GABA dramatically improves glucose tolerance in streptozotocin-induced diabetic rats fed with high-fat diet. Eur J Pharmacol, https://doi.org/10.1016/j.ejphar.2018.01.047 (2018).
Shang, W., Si, X., Zhou, Z., Strappe, P. & Blanchard, C. Wheat bran with enriched gamma-aminobutyric acid attenuates glucose intolerance and hyperinsulinemia induced by a high-fat diet. Food Funct 9, 2820–2828, https://doi.org/10.1039/c8fo00331a (2018).
Rangachari, M. & Kuchroo, V. K. Using EAE to better understand principles of immune function and autoimmune pathology. J Autoimmun 45, 31–39, https://doi.org/10.1016/j.jaut.2013.06.008 (2013).
McGinley, A. M., Edwards, S. C., Raverdeau, M. & Mills, K. H. G. Th17cells, gammadelta T cells and their interplay in EAE and multiple sclerosis. J Autoimmun. https://doi.org/10.1016/j.jaut.2018.01.001 (2018).
McRae, B. L., Vanderlugt, C. L., Dal Canto, M. C. & Miller, S. D. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med 182, 75–85 (1995).
Targoni, O. S. et al. Frequencies of neuroantigen-specific T cells in the central nervous system versus the immune periphery during the course of experimental allergic encephalomyelitis. J Immunol 166, 4757–4764 (2001).
McMahon, E. J., Bailey, S. L., Castenada, C. V., Waldner, H. & Miller, S. D. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med 11, 335–339 (2005).
Kuriyama, K. & Sze, P. Y. Blood-brain barrier to H3-gamma-aminobutyric acid in normal and amino oxyacetic acid-treated animals. Neuropharmacology 10, 103–108 (1971).
Bhat, R. et al. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci USA 107, 2580–2585 (2010).
White, H. S. Molecular pharmacology of topiramate: managing seizures and preventing migraine. Headache 45(Suppl 1), S48–56, https://doi.org/10.1111/j.1526-4610.2005.4501006.x (2005).
Porter, R. J., Dhir, A., Macdonald, R. L. & Rogawski, M. A. Mechanisms of action of antiseizure drugs. Handb Clin Neurol 108, 663–681, https://doi.org/10.1016/B978-0-444-52899-5.00021-6 (2012).
Sternberg, Z. et al. Acamprosate modulates experimental autoimmune encephalomyelitis. Inflammopharmacology 20, 39–48, https://doi.org/10.1007/s10787-011-0097-1 (2012).
Kalk, N. J. & Lingford-Hughes, A. R. The clinical pharmacology of acamprosate. Br J Clin Pharmacol 77, 315–323, https://doi.org/10.1111/bcp.12070 (2014).
Reilly, M. T. et al. Effects of acamprosate on neuronal receptors and ion channels expressed in Xenopus oocytes. Alcohol Clin Exp Res 32, 188–196, https://doi.org/10.1111/j.1530-0277.2007.00569.x (2008).
Wright, T. M. Tramiprosate. Drugs Today (Barc) 42, 291–298 (2006).
Gervais, F. et al. Targeting soluble Abeta peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol Aging 28, 537–547 (2007).
Aisen, P. S. et al. Tramiprosate in mild-to-moderate Alzheimer’s disease - a randomized, double-blind, placebo-controlled, multi-centre study (the Alphase Study). Arch Med Sci 7, 102–111 (2010).
Gauthier, S. et al. Effect of tramiprosate in patients with mild-to-moderate Alzheimer’s disease: exploratory analyses of the MRI sub-group of the Alphase study. J Nutr Health Aging 13, 550–557 (2009).
Tower, D. B. & Roberts, E. 562–578 (Pergamon Press, New York, 1960).
Jones, M. V., Sahara, Y., Dzubay, J. A. & Westbrook, G. L. Defining affinity with the GABAA receptor. J Neurosci 18, 8590–8604 (1998).
Van Gelder, N. M. & Elliott, K. A. Disposition of gamma-aminobutyric acid administered to mammals. J Neurochem 3, 139–143 (1958).
Loscher, W. & Frey, H. H. Transport of GABA at the blood-CSF interface. J Neurochem 38, 1072–1079 (1982).
Mendel, I., Kerlero de Rosbo, N. & Ben-Nun, A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur J Immunol 25, 1951–1959, https://doi.org/10.1002/eji.1830250723 (1995).
Tuohy, V. K., Lu, Z., Sobel, R. A., Laursen, R. A. & Lees, M. B. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. J Immunol 142, 1523–1527 (1989).
Hofstetter, H. H., Toyka, K. V., Tary-Lehmann, M. & Lehmann, P. V. Kinetics and organ distribution of IL-17-producing CD4 cells in proteolipid protein 139–151 peptide-induced experimental autoimmune encephalomyelitis of SJL mice. J Immunol 178, 1372–1378 (2007).
Bhandage, A. K. et al. GABA Regulates Release of Inflammatory Cytokines From Peripheral Blood Mononuclear Cells and CD4(+) T Cells and is Immunosuppressive in Type 1 Diabetes. EBioMedicine 30, 283–294, https://doi.org/10.1016/j.ebiom.2018.03.019 (2018).
Nusser, Z., Sieghart, W. & Somogyi, P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 18, 1693–1703 (1998).
Richerson, G. B. Looking for GABA in all the wrong places: the relevance of extrasynaptic GABA(A) receptors to epilepsy. Epilepsy Curr 4, 239–242, https://doi.org/10.1111/j.1535-7597.2004.46008.x (2004).
Farrant, M. & Kaila, K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res 160, 59–87, https://doi.org/10.1016/S0079-6123(06)60005-8 (2007).
Storustovu, S. I. & Ebert, B. Pharmacological characterization of agonists at delta-containing GABAA receptors: Functional selectivity for extrasynaptic receptors is dependent on the absence of gamma2. J Pharmacol Exp Ther 316, 1351–1359, https://doi.org/10.1124/jpet.105.092403 (2006).
Meera, P., Wallner, M. & Otis, T. S. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA(A) receptors. J Neurophysiol 106, 2057–2064, https://doi.org/10.1152/jn.00450.2011 (2011).
Naffaa, M. M., Hung, S., Chebib, M., Johnston, G. A. R. & Hanrahan, J. R. GABA-rho receptors: distinctive functions and molecular pharmacology. Br J Pharmacol 174, 1881–1894, https://doi.org/10.1111/bph.13768 (2017).
Chastain, E. M., Duncan, D. S., Rodgers, J. M. & Miller, S. D. The role of antigen presenting cells in multiple sclerosis. Biochim Biophys Acta 1812, 265–274, https://doi.org/10.1016/j.bbadis.2010.07.008 (2011).
Almolda, B., Gonzalez, B. & Castellano, B. Antigen presentation in EAE: role of microglia, macrophages and dendritic cells. Front Biosci (Landmark Ed) 16, 1157–1171 (2011).
Wheeler, D. W. et al. Anaesthetic impairment of immune function is mediated via GABA(A) receptors. PLoS One 6, e17152, https://doi.org/10.1371/journal.pone.0017152 (2011).
Lee, M., Schwab, C. & McGeer, P. L. Astrocytes are GABAergic cells that modulate microglial activity. Glia 59, 152–165, https://doi.org/10.1002/glia.21087 (2011).
Mead, E. L. et al. Microglial neurotransmitter receptors trigger superoxide production in microglia; consequences for microglial-neuronal interactions. J Neurochem 121, 287–301, https://doi.org/10.1111/j.1471-4159.2012.07659.x (2012).
Acknowledgements
We thank Marianne Cillufo for her help and advice, Ryan Chen, Bryan Tiu and other members of the Kaufman lab for their assistance. This work was supported in by grants from the National Multiple Sclerosis Society (to DLK), the National Institutes of Health (DK092480 to DLK, and AA021213 to RWO and MW), and DLK’s unrestricted funds. The authors thank UCLA’s Janis V. Giorgi Flow Cytometry Core Facility for performing cytometry.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: J.T., M.W., R.O., D.L.K. Performed the experiments: J.T., H.D. Analyzed the data: J.T., D.L.K. Wrote the paper: J.T., D.L.K. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
D.L.K. and J.T. are inventors of GABA and GABA-R related patents. All other authors have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tian, J., Dang, H., Wallner, M. et al. Homotaurine, a safe blood-brain barrier permeable GABAA-R-specific agonist, ameliorates disease in mouse models of multiple sclerosis. Sci Rep 8, 16555 (2018). https://doi.org/10.1038/s41598-018-32733-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-32733-3
Keywords
This article is cited by
-
Propofol maintains Th17/Treg cell balance in elderly patients undergoing lung cancer surgery through GABAA receptor
BMC Immunology (2022)
-
GAB functions as a bioenergetic and signalling gatekeeper to control T cell inflammation
Nature Metabolism (2022)
-
The Role of CD4+ T Cells in the Immunotherapy of Brain Disease by Secreting Different Cytokines
Journal of Neuroimmune Pharmacology (2022)
-
Homotaurine limits the spreading of T cell autoreactivity within the CNS and ameliorates disease in a model of multiple sclerosis
Scientific Reports (2021)
-
Canine distemper virus induces downregulation of GABAA,GABAB, and GAT1 expression in brain tissue of dogs
Archives of Virology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.