Abstract
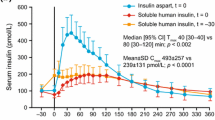
Insulin aspart is a novel rapid-acting insulin analogue with improved subcutaneous absorption properties when compared with soluble human insulin. Pharmacokinetic studies show an absorption profile with a time to reach peak concentration (tmax) about half that of human insulin, a peak plasma drug concentration (Cmax) approximately twice as high and shorter residence time. The potency and bio-availability of insulin aspart are similar to those of human insulin.
The pharmacokinetics of insulin aspart have been studied in healthy Caucasian and Asian-Japanese volunteers, in patients with type 1 and 2 diabetes mellitus, and in children with diabetes, with both pre- and postprandial administration and during continuous subcutaneous insulin infusion (CSII). The pharmacokinetic profile was similar to that of another rapid-acting insulin analogue, insulin lispro, on the basis of published information for that agent.
Pharmacodynamic studies show a smaller excursion of postprandial glucose with insulin aspart injected subcutaneously just before the meal compared with soluble human insulin injected 30 minutes before the meal in patients with type 1 diabetes mellitus, and an equivalent control in patients with type 2 diabetes displaying residual insulin production. In a treatment study, glucose excursions evaluated from 24-hour glucose profiles showed less variability with insulin aspart compared with human insulin. Adverse events, including hypoglycaemia-induced ventricular repolarisation and hypoglycaemic threshold and awareness, did not differ between insulin aspart and human insulin. The available data suggest that subcutaneous injections of insulin aspart just before meals better mimic the endogenous insulin profile in blood compared with human insulin, resulting in improved glucose control in a meal-related insulin regimen.
This review summarises the clinical pharmacokinetics and pharmacodynamics of insulin aspart in relation to human insulin and insulin lispro.











Similar content being viewed by others
References
Binder C. Absorption of injected insulin. Acta Pharmacol Toxicol 1969; 27 Suppl. 2: 1–84
Binder C, Lauritzen T, Faber O, et al. Insulin pharmacokinetics. Diabetes Care 1984; 1984: 188–99
Owens DR. Human insulin. In: DR Owens, editor. Clinical pharmacological studies in normal man. Lancaster: MTP Press Limited, 1986
Home PD. Insulin therapy. In: Alberti KGMM, Zimmet P, DeFronzo RA, editor. The international textbook of diabetes. 2nd ed. Chichester: Wiley, 1996: 899–928
Blundell T, Dodson G, Hodgkin D, et al. Insulin: the structure in the crystal and its reflection in chemistry and biology. Adv Protein Chem 1972; 26: 279–402
Brange J, Ribel U, Hansen JF, et al. Monomeric insulins obtained by protein engineering and their medical implications. Nature 1988; 333: 679–82
Kang S, Brange J, Burch A, et al. Subcutaneous insulin absorption explained by insulin’s physicochemical properties. Evidence from absorption studies of soluble human insulin and insulin analogues in humans. Diabetes Care 1991; 14(11): 942–8
Home PD, Lindholm A, Riis A, Insulin Aspart Study Group. Insulin aspart versus human insulin in the management of long-term blood glucose control in Type 1 diabetes: a randomized controlled trial. Diabet Med 2000; 17: 762–70
Raskin P, Riis A, Guthrie RA, et al. Use of insulin aspart, a fast-acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care 2000; 23: 583–8
Plum A, Agersø H, Andersen L. Pharmacokinetics of the rapid-acting insulin analog, insulin aspart, in rats, dogs, and pigs, and pharmacodynamics of insulin aspart in pigs. Drug Metab Dispos 1999; 28: 155–60
Kang S, Creagh FM, Peters JR, et al. Comparison of subcutaneous soluble human insulin and insulin analogues (AspB9, GluB27; AspB10; AspB28) on meal related plasma glucose excursions in Type 1 diabetic subjects. Diabetes Care 1991; 14: 571–7
Lutterman JA, Pijpers E, Netten PM, et al. Glycaemic control in IDDM patients during one day with injection of human insulin or the insulin analogues insulin X14 and insulin X14 (+Zn). Front Insulin Pharmacol 1993: 102-9
Wiefels K, Kuglin B, Hübinger A, et al. Insulin kinetics and dynamics in insulin-dependent diabetic patients after injection of human insulin or the insulin analogues X14 and X14 + Zn. Front Insulin Pharmacol 1993: 97-101
Heinemann L, Heise T, Jørgensen LN, et al. Action profile of the rapid acting insulin analogue: human insulin B28 Asp. Diabet Med 1993; 10: 535–9
Brange J, Vølund A. Insulin analogs with improved pharmaco-kinetic profiles. Adv Drug Deliv Rev 1999; 35: 307–35
Torlone E, Pampanelli S, Lalli C, et al. Effects of the short-acting insulin analog [Lys(B28), Pro(B29)] on postprandial blood glucose control in IDDM. Diabetes Care 1996; 19: 945–52
Kang S, Brange J, Burch A, et al. Absorption kinetics and action profiles of subcutaneously administered insulin analogues (AspB9, GluB27, AspB10, AspB28) in healthy subjects. Diabetes Care 1991; 14: 1057–65
Home PD, Barriocanal LA, Lindholm A. Comparative pharma-cokinetics and pharmacodynamics of the novel rapid-acting insulin analogue, insulin aspart, in healthy volunteers. Eur J Clin Pharmacol 1999; 55: 199–203
Kaku K, Urae A, Irie S, et al. Pharmacokinetics and pharmaco-dynamics of insulin aspart, a rapid-acting analog of human insulin, in healthy Japanese volunteers. Diabet Res Clin Pract 2000; 49: 119–26
Jacobsen LV. A bioequivalence study on insulin aspart. Novo Nordisk, 1998. (Data on file)
Lindholm A, Susaki T, Edwards A, et al. Dose dependency of insulin aspart in healthy Caucasians and Japanese [abstract]. Diabetes 2001; 50 Suppl. 2: A441
Lindholm A, McEwen J, Riis A. Improved postprandial glycemic control with insulin aspart. Diabetes Care 1999; 22: 801–5
Rosenfalck AM, Thorsby P, Kjems L, et al. Improved postprandial glycaemic control with insulin aspart in type 2 diabetic patients treated with insulin. Acta Diabetol 2000; 37: 41–6
Mortensen H, Olsen B, Lindholm A. Pharmacokinetics of a rapid-acting human insulin analogue, insulin aspart, in children and adolescents with Type 1 diabetes. Eur J Pediatr 2000; 159: 483–8
Halberg IB, Jacobsen LV, Dahl UL. A study on self-mixing insulin aspart with nph insulin in the syringe before injection [abstract]. Diabetes 1999; 48 Suppl. 1: A104
Brunner GA, Hirschberger S, Sendlhofer G, et al. Post-prandial administration of the insulin analogue insulin aspart in patients with type 1 diabetes mellitus. Diabet Med 2000; 17: 371–5
Home PD, Lindholm A, Hylleberg B, et al. Improved glycaemic control with insulin aspart: a multicentre randomized doubleblind cross-over trial in type 1 diabetes mellitus. Diabetes Care 1998; 21: 1904–7
Bode B, Strange P. Efficacy, safety and pump compatibility of insulin aspart used in continuous subcutaneous insulin infusion therapy in patients with type 1 diabetes. Diabetes Care 2001; 24: 69–72
Kang S, Creagh FM, Ara J, et al. Insulin analogues and human insulin: near-equivalent in vivo biological activity in healthy males in spite of widely different in vitro potencies [abstract]. Diabetes 1991; 40: 243A
Heinemann L, Kapitza C, Starke AAR, et al. Time-action profile of the insulin analogue B28Asp. Diabet Med 1996; 13: 683–4
Mudaliar SR, Lindberg FA, Joyce M, et al. Insulin aspart (B28 Asp-insulin): a fast-acting analog of human insulin. Diabetes Care 1999; 22: 1501–6
Heinemann L, Weyer C, Rauhaus S, et al. Variability of the metabolic effect of soluble insulin and the rapid acting insulin analogue insulin aspart. Diabetes Care 1998; 21: 1910–4
Frier BM, Ewing FME, Lindholm A, et al. Symptomatic and counterregulatory hormonal responses to acute hypoglycaemia induced by insulin aspart and soluble insulin in type 1 diabetes. Diab Metab Res Rev 2000; 16: 262–8
Harris ND, Robinson R, Ireland RH, et al. Comparative effects of human soluble insulin and the new insulin analogue, insulin aspart upon ventricular repolarization [abstract]. Diabetes 1999; 48 Suppl. 1: A114
Gammeltoft S, Hansen BF, Dideriksen LH, et al. Insulin aspart: a novel rapid-acting human insulin analogue. Exp Opin Invest Drugs 1999; 8: 1431–41
Drejer K. The bioactivity of insulin analogues from in vitro receptor binding to in vivo glucose uptake. Diab Metab Rev 1992; 8: 259–86
Kurtzhals P, Schäffer L, Sørensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000; 49: 999–1005
Andersen L, Vølund A, Olsen KJ, et al. Validity and use of a non-parallel insulin assay for pharmacokinetic studies of a rapid-acting insulin analog, insulin aspart. J Immunoassay. In press
Andersen L, Jørgensen PN, Jensen LB, et al. A new insulin immunoassay specific for the rapid-acting insulin analogue, insulin aspart, suitable for bioavailability, bioequivalence and pharmacokinetic studies. Clin Biochem 2000; 33: 627–33
Woodworth JR, Howey DC, Bowsher RR, et al. Comparative pharmacokinetics and glycodynamics of two human insulin mixtures. Diabetes Care 1994; 17: 366–71
Heinemann L, Ampudia-Blasco FJ. Glucose clamps with the Biostator: A critical appraisal. Horm Metab Res 1994; 26: 579–83
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214–23
Plum A, Larsen PS, Larsen UD, et al. Determination of in vitro plasma protein binding of insulin aspart and insulin detemir by equilibrium dialysis [abstract]. Diabetologia 1999; 42 Suppl. 1: A236
Duckworth WC. Insulin degradation: mechanisms, products, and significance. Endocrine Rev 1988; 9: 319–44
Striffler JS. Insulin clearance and microsomal glutathione-insulin transhydrogenase in perfused livers of fed and fasted rats. Diabetes Metab 1987; 13: 582–90
Thomas JH, Jenkins CDG, Davey PG, et al. The binding and degradation of 125I-labelled insulin by rat kidney brush-border membranes. Int J Biochem 1983; 15: 329–36
Lee VHL. Enzymatic barriers to peptide and protein absorption. Crit Rev Ther Drug Carrier Syst 1988; 5: 69–97
Clot JP. Characterization of insulin degradation products generated in liver endosomes: in vivo and in vitro studies [abstract]. FASEB J 1990; 4: A2115
Lauritzen T, Faber OK, Binder C. Variation in 125I-insulin absorption and blood glucose concentration. Diabetologia 1979; 17: 291–5
de Meijer PH, Lutterman JA, van Lier HJ, et al. The variability of the absorption of subcutaneously injected insulin: effect of injection technique and relation with brittleness. Diabet Med 1990; 7: 499–505
Kolendorf K, Bojsen J, Deckert T. Clinical factors influencing the absorption of 125I-NPH insulin in diabetic patients. Horm Metab Res 1983; 15: 274–8
Lindholm A, Home PD, Jacobsen LV. A comparison of pharmacokinetic and pharmacodynamic relationship of insulin aspart in normal subjects and people with diabetes. Diabetes 1999; 48 Suppl. 1: A357–8
Clauson PG, Linde B. Absorption of rapid-acting insulin in obese and non-obese NIDDM patients. Diabetes Care 1995; 18: 986–91
Plum A. Pharmacokinetic studies of insulin aspart in rat and pig. Novo Nordisk, 1999. (Data on file)
Howey DC, Bowsher RR, Brunelle RL, et al. [Lys(B28), Pro(B29)]-human insulin: a rapidly absorbed analogue of human insulin. Diabetes 1994; 43: 396–402
Howey DC, Bowsher RR, Brunelle RL, et al. [Lys (B28), Pro(B29)]-human insulin: effect of injection time on postprandial glycemia. Clin Pharmacol Ther 1995; 58: 459–69
Heinemann L, Heise T, Wahl LCH, et al. Prandial glycaemia after a carbohydrate-rich meal in type 1 diabetic patients: using the rapid acting insulin analogue [lys(B28), Pro(B29] human insulin. Diabet Med 1996; 13: 625–9
Wilde M, McTavish D. Insulin lispro: a review of its pharmacological properties and therapeutic use in the management of diabetes mellitus. Drugs 1997; 54: 597–614
Jacobson LV. A double-blind trial to investigate intra- and inter-individual variability in action profiles of insulin aspart. Novo Nordisk, 1998. (Data on file)
Marques JL, Georges E, Peacey SR, et al. Altered ventricular repolarisation during hypoglycaemia in patients with diabetes. Diabet Med 1997; 8: 648–54
Committee for Propriety Medicinal Products, The European Agency for the Evaluation of Medical Products. The assessment of the potential for QT interval prolongation by non-cardiovascular medicinal products. London: European Agency for the Evaluation of Medical Products, 1996. CPMP/986/96
Tattersall RB, Gill GV. Unexplained death of Type 1 diabetic patients. Diabet Med 1991; 8: 49–58
Tamas G, Marre M, Dedov I, et al. Improved glycaemic control with insulin aspart compared to human insulin using algorithm-driven dose optimization [abstract]. Diabetes 2000; 40 Suppl. 1: A127
Gale EAM. A randomized, controlled trial comparing insulin lispro with human soluble insulin in patients with Type 1 diabetes on intensified insulin therapy. Diabet Med 2000; 17: 209–14
Acknowledgements
The authors are employees of Novo Nordisk A/S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lindholm, A., Jacobsen, L.V. Clinical Pharmacokinetics and Pharmacodynamics of Insulin Aspart. Clin Pharmacokinet 40, 641–659 (2001). https://doi.org/10.2165/00003088-200140090-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200140090-00002