Article Text
Abstract
Objectives To assess whether social support or autonomy support intervention for patients with type 2 diabetes can achieve glycemic control at the end of intervention, and to test whether the glycemic control effect can be maintained for a long time.
Research design and methods In this cluster randomized controlled trial, 18 community healthcare stations (CHSs) were randomized to the following: (1) usual care group (UCG) offering regular public health management services, (2) social support group (SSG) providing 3-month social support intervention based on problem solving principles, and (3) autonomy support group (ASG) offering 3-month autonomy support intervention based on self-determination theory. A total of 364 patients registered in the CHSs were enrolled into either of the three groups. The primary outcome was hemoglobin A1c (HbA1c), and secondary outcomes were diabetes self-management (DSM) behaviors. Assessment was conducted at baseline and at 3 and 6 months.
Results Patients in ASG achieved better HbA1c reduction at the end of intervention (0.53% or 7.23 mmol/mol, p<0.001) than those in the UCG and successfully maintained it up to 6 months (0.42% or 5.41 mmol/mol, p<0.001). However, patients in SSG did not experience significant change in HbA1c at 3 or 6 months when compared with patients in UCG. Besides, patients in both the SSG (0.12, p<0.05) and ASG (0.22, p<0.001) experienced improvement in exercise at 3 months. Patients in ASG sustained improvement in exercise up to 6 months (0.21, p<0.001), but those in the SSG did not.
Conclusions Autonomy support for patients with type 2 diabetes could help achieve glycemic control at the end of intervention and successfully maintain it up to 6 months. These findings indicate that autonomy support has positive long-term effects on DSM behaviors and glycemic control and can be recommended in future diabetes intervention programs.
Trial registration number ChiCTR1900024354.
- patient self-management
- community-based research/intervention
- glycemic control
- behavior change
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Significance of this study
What is already known about this subject?
Effective diabetes self-management (DSM) can prevent or delay complications of diabetes by glycemic control, and DSM education and support programs can help patients with diabetes engage and maintain DSM behaviors. However, the effects faded as soon as the intervention program stopped.
What are the new findings?
Autonomy support for patients with type 2 diabetes could help achieve glycemic control at the end of 3 months of intervention and successfully maintain it up to 6 months, and autonomy support has positive long-term effects on DSM behaviors and glycemic control.
How might these results change the focus of research or clinical practice?
To maintain DSM behaviors of patients with diabetes, autonomy support can be recommended in future diabetes intervention programs.
Introduction
Type 2 diabetes, along with the other three major non-communicable diseases (NCDs), accounts for over 80% of all premature NCD deaths.1 According to the most recent estimates from the International Diabetes Federation, 425 million adults aged between 20 and 79 years were estimated to have diabetes for the year 2017, and the number is expected to increase to 629 million by 2045.2 Diabetes has become a recognized public health concern in the 21st century.
Effective diabetes self-management (DSM) can prevent or delay complications of diabetes by glycemic control.3 DSM behaviors, including diet, exercise, medication management and glucose monitoring, were strongly recommended by both American and Chinese national guidelines.4 5 To encourage patients with diabetes to adopt and maintain DSM behaviors, DSM education is the critical element of care. Given that self-management is a lifelong effort, it does not stop when patients leave the educator’s office, and DSM support is recommended to be an ongoing process.4 In DSM support programs, social support from peers and family could help patients overcome barriers and encourage them to engage in DSM behaviors, and then work on improving DSM behaviors and clinical outcomes at the end of intervention.6–9 However, the effects faded as soon as the intervention program stopped.10 11 Maintenance of sustainable DSM behaviors and glycemic control is one of the challenges of the DSM support program.
According to the self-determination theory (SDT), encouraging persistent behavior changes in patients not only requires the knowledge and skills for change but also needs patients’ autonomous motivation and self-determination.12 13 Unfortunately, many patients failed to maintain long-term behavior changes because they lacked autonomous motivation.12 With autonomous motivation, patients will perceive themselves to be the initiator of their behavior change; their health-related behaviors are more likely to be internalized; and thus, behavior changes will be better maintained.14 In an autonomy-supportive atmosphere, patients’ autonomous motivation for change is stronger than in the situations where people feel pressured to change by others.15 Consequently, autonomy support was recommended in healthcare practice.16 17 A study showed that autonomous motivation played a mediating role between perceived autonomy support and patient’s DSM behaviors.18 It is likely that offering autonomy support to patients could foster a patient’s autonomous motivation, which could in turn lead patients to adopt and maintain DSM behaviors, achieve better glycemic control, and maintain the effect for a longer time.
The aim of this study was to evaluate the effects of autonomy support intervention for patients with type 2 diabetes and to provide further evidence of the effects of autonomy support on long-term DSM behavior change and glycemic control. The hypotheses of this study are as follows: (1) both autonomy support and social support for patients with type 2 diabetes could achieve DSM behavior change and glycemic control at the end of intervention, and (2) the effects of autonomy support intervention could maintain the behavior change and glycemic control for a longer time.
Research design and methods
Design overview and participants
The trial was performed as a cluster randomized controlled trial (RCT) with three arms (six clusters in each arm) and the observation time was 6 months. Randomization clusters were 18 community healthcare stations (CHSs) from six community healthcare centers (CHCs), Beijing, China (figure 1). Chinese CHCs are set up according to subdistrict boundaries, and each CHC has several CHSs to provide healthcare service for the neighborhood residents. Generally, patients registered in the same CHS are familiar with each other and receive the usual care from the same community doctor. In this case, the risk of contamination effects is too high to achieve the patient-level randomization. Hence, a cluster RCT was conducted, and the patients registered in the same CHS were considered as a cluster.
Site selection and randomization. ASG, autonomy support group; CHC, community healthcare center; CHS, community healthcare station; SSG, social support group; UCG, usual care group.
The potential participants were from a cross-sectional study conducted earlier, which was the pilot study of this trial. The aim of the pilot study was to identify the influencing factors of DSM behaviors. The eligible patients in this study were 18 years old and above and diagnosed with type 2 diabetes by medical institutions for at least 3 months. Patients with dementia or psychotic illnesses were excluded. Patients living alone were excluded from the trial because the intervention needed the participation of family members.
Recruitment
Recruitment was conducted by community doctors from April to May 2017. Recruitment commenced with posters at community public places and was followed by telephone recruitment based on the patients’ healthcare record. At the end of the pilot study, patients interested in the intervention study were enrolled as candidates of the trial. A random number generated by the computer was given to each candidate within a CHS. Telephone recruitment was conducted according to the order of the random number until 20 participants were recruited in each CHS. Twenty patients in the same CHS formed one cluster of the trial. All the participants in each CHS were invited to an information meeting, and the trial information was delivered, including research purpose, research content, intervention methods, benefits and risks of participating in the trial. Five patients (1.4%) did not come to the information meeting, and nine patients (2.5%) volunteered to participate but were not randomly selected. The specific participants in each cluster were presented in online supplementary table S1. All the participants signed the informed consent, and the trial was registered.
Supplemental material
Randomization
To control the confounders at community level, block randomization was used. Three clusters from the same CHC were randomly allocated to usual care group (UCG), social support group (SSG) and autonomy support group (ASG) by using random digits (figure 1). Randomization took place before the recruitment. Masking was not used in participants or researchers due to the need for training and intervention. In order to control observational bias, data collectors were blinded throughout the trial.
Interventions
Usual care group
According to essential public health services for patients with type 2 diabetes in China, each patient can receive face-to-face DSM education from the community doctor every 3 months. DSM education focuses on diabetes knowledge, including diabetes diet, exercise, medication, glucose monitoring and complication prevention. Besides, each participant in UCG also received a booklet about DSM knowledge from the research team. UCG served as the control group in this study.
Social support group
On the basis of intervention in UCG, patients in SSG also received social support from the community doctors, peer leaders and family. The support interventions for SSG focused on problem solving, including eight sessions, overview of diabetes, diet, exercise, glucose monitoring, medication management, emotional management, and reinforcing the diet and exercise behavior. In each session, the community doctors conducted DSM education focusing on one topic and delivered a goal and action plan for the corresponding topic for the patients, which was then followed by the DSM support. The participants were encouraged to share their barriers and solutions in the action plan implementation, and then, a group discussion focusing on problem solving was led by peer leaders. A handbook was given to family members on how to offer support to patients with diabetes. Each session lasted 60 min and the whole intervention process took 3 months. Sessions were conducted once a week in the first month and twice a week in the second and third months.
Autonomy support group
The same interventions as in SSG were provided to patients in ASG, but both DSM education and support were provided using a different approach. According to the SDT, the key point of autonomy support is to foster patient motivation for DSM behaviors by meeting three basic psychological needs of the patients, including sense of autonomy, competence and relatedness.12 19 Therefore, the intervention activities were conducted as follows: (1) to satisfy the patient’s sense of autonomy, the DSM education stressed why the DSM behaviors should be undertaken, and an optional list of DSM behaviors was offered to the patients; (2) the community doctor guided the patients to conduct a self-evaluation, and then set one’s own special target of behavioral change and an action plan with a self-determined approach; (3) to make sure support was offered based on patients’ needs, patients were encouraged to find out what kind of support they needed from their family or peers; (4) to improve competence, patients learned and reinforced the essential DSM skills in each session; when facing obstacles in conducting the action plan, they could put forward the solutions or they could seek advices from peers; and (5) to provide patients with a sense of relatedness, the doctors, family members, and peer leaders were to minimize the pressure and acknowledge patients’ feelings and perspectives while providing support. A warm interpersonal environment was to be provided during the whole process. The number and topics of sessions in ASG were the same as those in SSG. The interventions for patients in the three groups are shown in online supplementary table S2.
Outcomes and follow-up
The primary outcome was hemoglobin A1c (HbA1c). The nurses working in the CHSs obtained consent and collected blood using standardized methodology, and all the blood samples were tested by a professional testing agency. The secondary outcomes were DSM behaviors, including diet, exercise, medication management and glucose monitoring. DSM behaviors were measured by Diabetes Self-management Knowledge, Attitude, and Behavior Assessment Scale (DSKAB-SF), which was developed by the Chinese Center for Disease Control and Prevention20 with the Cronbach's α coefficient being 0.83 among the patients with diabetes in the Chinese community.21 Seven items from DSKAB-SF (online supplementary table S3) were used to measure DSM behavior of patients with type 2 diabetes in the past 3 months, and the data were collected by questionnaires. High behavior scores represent better behaviors. The intervention phase lasted for 3 months, and data were collected at baseline and at 3 and 6 months.
Statistical analysis
The trial tested the main effect (HbA1c) change of UCG versus SSG, UCG versus ASG, and SSG versus ASG. The minimum necessary sample size was 94 participants for each arm based on an SD for HbA1c of 1.0%,22 23 80% power to detect a difference of 0.57%24 in mean HbA1c for each main effect, intracluster correlation of 0.057 and an average cluster size of 20 participants. Assuming a 20% loss of participants to follow-up, 113 participants were needed in each arm.
To describe and compare characteristics of participants among the three groups, continuous variables were presented as mean±SD and were compared with Student t-test, and categorical variables were presented as proportions and were compared with Pearson χ2 test. To test the effects of interventions on each follow-up time point, linear mixed model was used. Considering the cluster structure of the data, clustering variables was included in random model. In the fixed model, we included the study group, time, a study group×time interaction, the baseline variable, and the potential demographic confounders. To test the relationship between DSM behaviors and glycemic control, the multilevel regression was applied.
Missing data treatment
Imputing of missing data of the baseline were by group mean imputing,25 and this method was used to deal with two missing HbA1c data at baseline. As the linear mixed model allows the participants to be analyzed if they had data at one or more time points,26 we did not deal with the missing data at the follow-up of 3 or 6 months. To investigate the impact of missing data, we also conducted an additional sensitivity analysis on completer sample (online supplementary table S4). As there was no change in the results, the unadjusted results were reported.
We followed the Consolidated Standards of Reporting Trials (CONSORT) statement27 and its extension to clusters randomized trials28 for analyses and reporting.
Results
Participant flow and basic characteristics
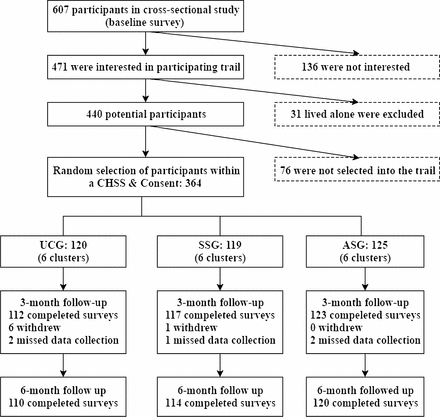
The CONSORT diagram (figure 2) shows the recruitment and follow-up in this study. Of the 607 participants who were invited in the cross-sectional study, 440 (72.5 %) were interested and met the inclusion criteria in the trial. The final analysis set comprised 64 patients: 120 in UCG, 119 in SSG, and 125 in ASG. The follow-up rate was 94.5% and met expectations. The actual intracluster correlation with a mean cluster size of 20.2 was calculated to be 0.025. Overall, the baseline characteristics were well matched among the three groups (table 1).
Basic characteristics of participants
Recruitment and follow-up of participants. ASG, autonomy support group; CHSs, community healthcare stations; SSG, social support group; UCG, usual care group.
Glycemic control
The HbA1c changes in the three groups over time are shown in table 2, and HbA1c trajectories for each group are also shown in online supplementary figure 1. The participants in SSG achieved a reduction in HbA1c at 3 months (−0.19% or −2.11 mmol/mol, p<0.05) but failed to sustain it over 6 months. However, the clinical improvement in SSG at 3 months was not statistically different when compared with that of UCG. In ASG, participants experienced an HbA1c reduction in the 3-month follow-up (−0.66% or 5.75 mmol/mol, p<0.001) and successfully maintained the effect at the 6-month follow-up (−0.50% or 4.64 mmol/mol, p<0.001).The hypoglycemic effects in ASG were −0.53% (-7.23 mmol/mol) at 3 months and −0.42% (−5.41 mmol/mol) at 6 months compared with those in the UCG (table 2). Similarly, hypoglycemic effects in the ASG were −0.47% (−5.10 mmol/mol) and −0.47% (−5.11 mmol/mol) when compared with those in the SSG at the follow-ups of 3 and 6 months, respectively.
Changes in HbA1c and diabetes self-management behaviors over time
DSM behavior change
A slight improvement of diet behavior was found at the 6-month follow-up among the three groups, but there was no difference between different groups. The exercise score of patients in UCG experienced a decline (−0.11, p<0.01) at 3 months, while that of those in SSG did not change. The exercise score of patients in ASG improved in 3 months, and this improvement was maintained at 6 months. Compared with UCG, the exercise improvements achieved by patients in ASG at the follow-ups of 3 and 6 month were 0.22 (p<0.001) and 0.21 (p<0.001), respectively. Moreover, the exercise improvements the of the patients in ASG were statistically significant (0.10 and 0.20 at the follow-ups of 3 and 6 months, respectively) when compared with those of patients in the SSG. Among the three groups, the medication behavior did not change over time, and there was no difference between the groups. The glucose monitoring score of the patients in the SSG and ASG increased 0.08 (p<0.001) and 0.09 (p<0.001), respectively. However, this change did not sustain at the 6-month follow-up and did not show a statistical significance between the groups.
To get a better understanding of the relationship between the DSM behaviors and glycemic control, a multilevel regression model of DSM behaviors on HbA1c reduction was conducted (table 3). The result shows that both diet and exercise behavior change were positively related to the HbA1c reduction.
Multilevel regression model of the effects of diabetes self-management behaviors on hemoglobin A1c reduction
Conclusions
The results tend to support hypotheses that autonomy support for patients with type 2 diabetes can achieve DSM behavior change and HbA1c reduction at the end of intervention and maintain the effects for a longer time. The results of the intergroup comparison showed that patients in ASG achieved HbA1c reduction and exercise improvement at the end of intervention, and successfully maintained it up to 6 months when compared with those in the UCG or SSG. Compared with patients in the UCG, patients in SSG did not experience HbA1c reduction at 3 or 6 months. In addition, the result from the regression model shows that there is a positive relationship between DSM behavior change and HbA1c reduction, which indicates that the effects of interventions on glycemic control might through DSM behavior change.
Patients in ASG experienced exercise improvement and HbA1c reduction at the end of intervention and maintained the effects at 6 months. These changes could be explained by the SDT model of health behavior change.12 In the autonomy support interventions, patients experienced a feeling of autonomy, competence and relatedness, and their motivation for health-related behaviors was internalized, which led patients to engage and maintain the DSM behaviors, and thus achieve glycemic control. The HbA1c reduction and exercise improvement in ASG could be maintained until 6 months, and this lasting effectiveness might be due to the enhancement of the patient’s autonomous motivation. In healthcare, patients perceived autonomy support can predict autonomous motivation to participate in medical treatment,13 and this autonomous participation will maintain patients’ behavior change and achieve long-term positive health outcome.13 29 Autonomy support interventions were applied in the treatment of other health issues (such as improving physical activity,30 smoking cessation,31 and substance abuse treament,32 and were found to have long-term effects. When offering autonomy support for patients with type 2 diabetes, similar long-term effects were also found in this study. Hence, pieces of evidence from current and previous studies illustrate that autonomy support has a unique effect in maintaining long-term medical treatment.
However, compared with UCG, patients in SSG experienced exercise improvement at the end of intervention but failed to maintain it at 6 months. Besides, no statistically significant HbA1c reduction was found in patients in SSG. These results indicate that social support might have a short-term effect on DSM behavior change, and it is hard to maintain the effect over a longer period of time. Similar results were also found in other community-based DSM support intervention studies.10 33
In the current study, results from regression analysis showed that both diet and exercise behavior modification were related to HbA1c reduction, which indicated that the interventional effects on glycemic control might be mediated through DSM behaviors, and this mediating effect was supported by a previous study.14 Four DSM behaviors were observed in this study; only improvement in exercise was found. Thus, the glycemic control effects might be beneficial from exercise improvement. A previous study showed that the intervention program based on SDT could promote exercise18 34 and diet behavior,35 but diet behavior improvement effect was not found in the current study. One of the reasons could probably be a lack of family diet support. Diabetic diet needs the cooperation of families, such as a family diabetes meal plan.9 As family training was only arranged during the first session, family diet support for diabetes might not be enough in this study.
Some limitations need to be mentioned for this study. First, the trial was conducted in Chinese urban communities with high economic status, so the community doctors were likely to have higher professional competence to conduct the interventions. Professional ability of community doctors is an influencing factor of diabetes healthcare quality.36 When this intervention is applied in rural areas, training for community doctors must be emphasized. Second, this kind of community-based autonomy support intervention is better suited for older individuals. The average age of current sample was 65.14±7.23 years old and 75% of them were older than 60 years. Participants who are younger than 60 years are more likely to be lost to follow-up because they have to work and might miss the intervention session. Third, we failed to collect the individual intervention data, so the intervention dosage effect should be explored in a future study.
This cluster RCT showed that autonomy support for patients with type 2 diabetes could achieve improvement in exercise behaviors and glycemic control at the end of intervention, and these effects could be successfully maintained up to 6 months. These findings also indicate that autonomy support can help maintain glycemic control effect for a longer time, which can be recommended in future diabetes intervention programs.
Acknowledgments
The authors thank the staff from the Center for Disease Control and Prevention in Chaoyang and Dongsheng Districts, Beijing, China, who gave a lot of support in the implementation of this research. The authors also thank the patients and their families who participated in this study.
References
Footnotes
Contributors SL and CC conceived of the study concept and design. QY, YJ and YS analyzed data. QY wrote the manuscript, and YJ contributed to the discussion. XJ and XF reviewed the manuscript. XJ, XF and JL took responsibility for collecting the data and quality control of the data. CC and QY are the guarantors for this study, had full access to all the data and took the responsibility for the integrity of the data and the accuracy of the data analysis.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval Ethics approval (IRB00001052-17017) was obtained from the institutional review board of Peking University Health Science Center.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request. The raw data of the current study cannot be shared at this time because the data is part of an ongoing study, but the data are available from the corresponding author on reasonable request.