Article Text
Abstract
Albuminuria is useful for early screening and diagnosis of kidney impairment, especially in people with pre-diabetes or type 2 diabetes (T2D), which is the leading cause of chronic kidney disease (CKD) and end-stage kidney disease (ESKD), associated with increased mortality, poor cardiovascular outcomes, and high economic burden. Identifying patients with CKD who are most likely to progress to ESKD permits timely implementation of appropriate interventions. The early stages of CKD are asymptomatic, which means identification of CKD relies on routine assessment of kidney damage and function. Both albuminuria and estimated glomerular filtration rate are measures of kidney function. This review discusses albuminuria as a marker of kidney damage and cardiorenal risk, highlights the importance of early screening and routine testing for albuminuria in people with T2D, and provides new insights on the optimum management of CKD in T2D using albuminuria as a target in a proposed algorithm. Elevated urine albumin can be used to detect CKD in people with T2D and monitor its progression; however, obstacles preventing early detection exist, including lack of awareness of CKD in the general population, poor adherence to clinical guidelines, and country-level variations in screening and treatment incentives. With albuminuria being used as an entry criterion and a surrogate endpoint for kidney failure in clinical trials, and with novel treatment interventions available to prevent CKD progression, there is an urgent need for early screening and diagnosis of kidney function decline in people with T2D or pre-diabetes.
- diabetes mellitus, type 2
- diabetic neuropathies
Data availability statement
No data are available. The data supporting the findings of this study are not currently available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Type 2 diabetes (T2D) is the leading cause of chronic kidney disease (CKD) and end-stage kidney disease (ESKD).1–3 More than one-third of people with T2D also have CKD,4 and this population is associated with a 10-fold or greater increase in all-cause mortality compared with T2D alone.5 Furthermore, CKD progression leads to ESKD, which is irreversible and fatal in the absence of kidney replacement therapy.6 CKD and ESKD are associated with high economic burden, accounting for 22.3% (US$81.8 billion) and 7.2% (US$36.6 billion), respectively, of all Medicare fee-for-service spending in 2018.7 8 Medicare expenditures for people with CKD have risen at a rate higher than expenditures for the general Medicare population and have been found costlier for people with CKD and comorbid heart failure or diabetes (type 1 or 2), highlighting clear clinical and economic rationales for early identification and treatment intervention to limit CKD progression in all populations, particularly in people with T2D and cardiovascular risk factors.7
Early diagnosis of CKD may prove vital in people with T2D, allowing for more immediate clinical care, and identification of the risks for progression to ESKD. This may be particularly important in Afro-Caribbean/African-American, Hispanic and Asian populations, who tend to have a higher risk of CKD progression relative to White people; the increased risk may be explained in part by factors such as higher blood pressure and increased prevalence of T2D and cardiovascular disease in these populations.9 However, diagnosis of CKD is challenging because of the lack of symptoms during its early stages.10 Other potential factors that hinder early detection of CKD include a lack of awareness of CKD in the general population, poor adherence to clinical guidelines, and the variation in screening and treatment incentives by practice and by country.10
Diagnosis and determination of the severity of CKD relies on routine measurement of reduced estimated glomerular filtration rate (eGFR; <60 mL/min/1.73 m2) for at least 3 months and/or persistently elevated albuminuria (urine albumin-to-creatinine ratio (UACR)), to assess the overall kidney function and the presence of kidney damage, respectively.1 2 11 As such, the Kidney Disease: Improving Global Outcomes (KDIGO) CKD working group uses a combination of eGFR and UACR categories in its risk stratification tool for predicting CKD outcomes (figure 1).2 Clinical guidelines in Europe, USA and other countries recommend yearly screening of albuminuria and eGFR in people with T2D.11 12 However, real-world data suggest that rates of albuminuria testing in clinical practice are suboptimal,4 resulting in a gross underestimation of the risk of CKD in people with T2D. Routine assessment of both screening for CKD prognosis and management was further emphasized in a recent systematic review that showed an association between the prevalence of comorbidities in CKD and an increase in albuminuria severity.13 While CKD is common, only a small proportion of patients were classified as high risk or very high risk according to the KDIGO classification, highlighting key gaps in the burden and outcomes of CKD defined by the KDIGO 2012 guideline.13 With novel treatment interventions now available that can slow CKD progression, there is an urgent need for the early screening and diagnosis of kidney function decline in people with T2D. To this end, this review discusses albuminuria as a marker of kidney damage and cardiorenal risk, and the importance of earlier and more frequent screening.
Prognosis of CKD by GFR and albuminuria categories. Green: low risk (if no other markers of kidney disease, no CKD); yellow: moderately increased risk; orange: high risk; red: very high risk. Reproduced with permission from Kidney Disease: Improving Global Outcomes (KDIGO) 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease.1 A, albuminuria category; CKD, chronic kidney disease; G, GFR category; GFR, glomerular filtration rate.
Albuminuria as a marker of kidney damage and cardiorenal risk
UACR can be easily measured by spot urine samples, preferably obtained from the first morning void.2 Despite strong recommendations to screen for UACR in patients with T2D, studies show testing for UACR is underused compared with eGFR testing: the Awareness, Detection and Drug Therapy in Type 2 Diabetes and Chronic Kidney Disease study reports 85% of patients undergo eGFR testing versus 47% with UACR determination14; similar patterns for underutilization of eGFR and UACR testing were noted in the Center for Kidney Disease Research, Education, and Hope (CURE-CKD) registry of people with T2D at risk for CKD.15 16 Addressing this discord in eGFR and UACR testing is important because detectable increases in albuminuria generally occur before a decline in eGFR and early changes could indicate signs of kidney disease.17 18 Similarly, albuminuria has been associated with an elevated risk of hospitalization in older adults with and without diabetes (type 1 or 2), further emphasizing the importance of albuminuria measurement as a marker.19
As well as being useful for the early detection of CKD in people with T2D, elevated UACR levels can also be used to monitor CKD progression.2 The ability of albuminuria to predict the progression of kidney disease is irrespective of eGFR,20 with the relative risks of ESKD, acute kidney injury, and progressive CKD increasing with elevating levels of albuminuria.17 20 21 In general, lower eGFR and higher levels of albuminuria (>300 mg/g) independently predict faster progression to ESKD among patients with stage 3 CKD (eGFR 30–60 mL/min/1.73 m2).20 While kidney function decline is a continuum, recognized albuminuria categories in CKD include: A1 (normal to mildly increased albuminuria), defined as UACR <30 mg/g; A2 (moderately increased albuminuria), defined as UACR 30–300 mg/g; and A3 (severely increased albuminuria), defined as UACR >300 mg/g (figure 1).2
Large interindividual variability in kidney decline has been documented. The UK Prospective Diabetes Study found that 38% of people with T2D developed albuminuria and 29% developed renal impairment, with many developing one outcome but not the other.22 Thus, some patients with T2D may have CKD without the presence of albuminuria,22 suggesting that the assessment of albuminuria alone may not optimally identify people with T2D who are at high risk of kidney impairment. An additional challenge in screening for CKD in people with T2D is the occurrence of hyperfiltration with normal creatinine/eGFR and without albuminuria. Results from a large observational study indicate that in T2D, both hyperfiltration and high-normal eGFR levels are associated with increased risk of mortality, independent of confounders that may directly impact mortality or GFR estimation.23 Routine assessment of both albuminuria and eGFR is warranted in these patients. Also, it is important to note that elevated albumin in the urine may be indicative of other disorders. Albuminuria is the earliest marker of other glomerular diseases, and may be elevated in hypertensive nephrosclerosis; it is also associated with hypertension, obesity, and vascular disease.2 Furthermore, exercise within 24 hours of albuminuria assessment, infection, fever, congestive heart failure, marked hyperglycemia, menstruation, and marked hypertension may elevate the UACR independent of kidney damage.11
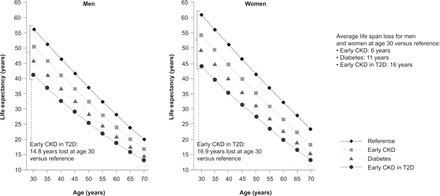
While elevated albuminuria can be indicative of CKD, incremental increases in albuminuria are associated with corresponding increases in cardiorenal risk.24 Concomitant CKD and T2D is an independent risk factor for cardiovascular events, with this population being approximately three times more likely to die from cardiovascular-related causes than those with T2D alone, and almost six times more likely to die from cardiovascular-related causes than those with neither T2D nor CKD.5 Based on a report from the Framingham Heart Study, the presence of albuminuria is associated with a higher risk of incident heart failure, specifically heart failure with reduced ejection fraction, in the general population. In patients with T2D and kidney disease, patients with an increased level of baseline albuminuria had a higher risk for heart failure compared with those with low albuminuria. In addition, the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) study showed that reduction in albuminuria by 50% was associated with reduced risks of cardiovascular events by 18% and heart failure by 27%.24 People with T2D and moderately to severely increased albuminuria have an approximately threefold, fourfold, and fivefold greater risk of myocardial infarction, cardiovascular death and hospitalization for heart failure, respectively, than those with T2D alone.25 A recent study (n=512 700) showed that early CKD in people with diabetes may shorten life expectancy by 16 years, compared with 6 years for early CKD without diabetes (either type 1 or type 2), and 10 years for diabetes alone (either type 1 or type 2; figure 2).26 Patients with high albuminuria may die of cardiovascular events before developing ESKD.20
Life expectancy in people with early CKD, T2D, and early CKD in T2D. The reference group consisted of participants with neither diabetes nor CKD. Early CKD was defined as CKD stages 1–3 without diabetes. Diabetes was defined as T2D without CKD. Early CKD in T2D was defined as diabetes with early CKD stages 1–3. Reproduced with permission from Kidney International.26 CKD, chronic kidney disease; T2D, type 2 diabetes.
Treatment and prevention of CKD
The ability to identify patients with CKD most likely to progress to ESKD would permit timely implementation of appropriate interventions. Progression of CKD poses a significant impact on the health and quality of life of affected patients,27 28 as well as a large financial burden.7 Efforts to improve care of patients with CKD should focus on reducing the incidence of kidney failure (often defined as a composite of outcomes including sustained low/declining GFR and clinical outcomes of transplantation, dialysis or death from kidney failure), and should provide more affordable treatment options, as well as improving access to kidney transplantation and home dialysis.27 28
Early intensive glycemic control can reduce the risk of developing high albuminuria by one-third compared with the standard of care in people with type 1 diabetes29 and significantly reduce the rate of progression from normoalbuminuria to microalbuminuria in newly diagnosed T2D.30 Current pharmacologic recommendations for managing CKD in T2D include reducing hyperglycemia and hypertension, reducing cardiovascular risk through management of dyslipidemia with statin therapy, using an ACE inhibitor or angiotensin receptor blocker (ARB) and most recently, using sodium-glucose co-transporter-2 inhibitors (SGLT2is) or glucagon-like peptide-1 receptor agonists (GLP-1RAs).2 ACE inhibitors and ARBs are not recommended for the primary prevention of CKD; however, they are recommended as separate treatments to help preserve kidney function in all people with T2D and persistent albuminuria (ie, persistent UACR >30 mg/g).1 2 ACE inhibitors and ARBs are potent antihypertensive agents that counter the vasoconstrictive effects of angiotensin II, causing selectively greater vasodilatation of the efferent arterioles of the glomeruli, thereby reducing intraglomerular pressure.1 ACE inhibitors and ARBs have also been shown to provide kidney protection independent of blood pressure control.31 32
SGLT2is and GLP-1RAs have demonstrated reduced risk of CKD progression in people with T2D in several clinical trials.33–38 Improved kidney outcomes in people with T2D, eGFR <60 mL/min/1.73 m2, and either established cardiovascular disease or cardiovascular risk factors,37 and reduced risk of kidney failure and cardiovascular events in patients with CKD regardless of T2D status,38 39 have resulted in SGLT2is being the recommended first-line glucose-lowering medication, with or without metformin and regardless of glucose control levels in people with CKD and T2D when eGFR is ≥30 mL/min/1.73 m2 and UACR is >300 mg/g.1 40
A proposed mechanism for SGLT2is is improving the intrarenal hemodynamics, which protects the glomeruli from high-pressure damage. This occurs via tubuloglomerular feedback from the macula densa, leading to constriction of the afferent arteriole or dilation of the efferent arteriole.41 Novel approaches to slowing the progression of CKD in people with T2D involve the pharmacologic blockade of mineralocorticoid receptor (MR) overactivation in the kidney.42 MR overactivation is known to exacerbate inflammation and fibrosis in the kidneys, the heart, and the vascular system.42 Finerenone, a novel, selective, non-steroidal, MR antagonist that blocks MR overactivation, has demonstrated significant dose-dependent reductions in albuminuria in people with T2D and CKD,43 as well as significantly reducing the risk of kidney failure, sustained ≥40% decline in eGFR, or renal death (composite endpoint), and other cardiovascular outcomes in people with CKD and T2D in Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD).44 In addition, UACR reductions were demonstrated with finerenone versus placebo (−34.7% vs −4.7%).44 Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD), a recently completed successful companion phase III trial to FIDELIO-DKD, met its composite primary endpoint of cardiovascular death and non-fatal cardiovascular events versus placebo in people with CKD and T2D.44 45 Despite also showing reduced albuminuria in people with T2D and CKD,1 the steroidal MR antagonists spironolactone and eplerenone are currently underused because of difficulties in managing severe hyperkalemia and worsening of kidney function.46
Ethnic minority representation varies across these clinical trials but is consistently low compared with Caucasian representation; this is not CKD-specific but a global and societal issue that needs to be addressed across all forms of healthcare. For example, African Americans account for 35% of people with CKD,47 yet in large CKD studies such as Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE), Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD), and FIDELIO-DKD, these patients accounted for 5%, 4%, and 5% of the trial population, respectively.38 39 44
Assessment of albuminuria is an important component of entry criteria for clinical trials, and its use as a predictor of efficacy should be assessed. Clinical trials in people with CKD and T2D typically include UACR measurements as part of the study entry criteria; however, criteria often vary hugely between studies.38 39 43 44 A recent meta-analysis supported the role of albuminuria as a surrogate endpoint in clinical trials of progression of CKD, with a reduction in albuminuria of >30% associated with substantial reductions in the risk of ESKD.48 Proteinuria may contribute to worsening kidney function by overloading the tubular epithelial cells leading to intrarenal activation of complement, resulting in interstitial inflammation predominantly mediated by macrophages and sustained fibrogenesis.49 As such, remission of albuminuria (defined as reversal of UACR to normoalbuminuria or <30 mg/g creatinine and a reduction in UACR by ≥30% from baseline at two consecutive time points) was used as the primary endpoint in the Esaxerenone (CS-3150) in Patients with Type 2 Diabetes and Microalbuminuria trial, which investigated the use of esaxerenone, a non-steroidal MR antagonist.50 Despite evidence for the utility of albuminuria, regulatory authorities place more emphasis on GFR thresholds over UACR, currently accepting a 30%–40% GFR decline as a surrogate endpoint for kidney failure in clinical trials of kidney disease progression. However, this may not be appropriate for drugs targeted at early stages of CKD or with potential hemodynamic effects.17
Albuminuria as a target for intervention
Microalbuminuria (UACR <300 mg/g) has been reported in association with impaired fasting glucose and impaired glucose tolerance,51 with a prevalence of 15.5% in people with pre-diabetes.51 Correlation between the presence of microalbuminuria in people with pre-diabetic conditions and progression towards T2D suggests that screening of albuminuria in the general population may be beneficial for the early prevention of kidney damage.51
Reducing albuminuria may slow progression of CKD and should, therefore, be considered as a separate target for kidney-protective therapy. A meta-analysis of data from 41 clinical trials that included participants with diabetes (71%), glomerular disease (4.4%) and other CKD (25.2%) demonstrated that decreasing albuminuria is associated with a reduced risk for developing the clinical endpoint (a composite of ESKD, doubling of serum creatinine concentration, or eGFR <15 mL/min/1.73 m²), predictive of CKD progression.48 This is in addition to monitoring cardiovascular risks using well-established surrogate endpoints, such as glycated hemoglobin for glucose lowering, blood pressure for control of hypertension, and low-density lipoprotein cholesterol for efficacy of lipid lowering.52
Optimum patient management requires regular screening of people with T2D to ensure early identification of CKD, followed by targeted intervention to reduce albuminuria (ie, with the use of SGLT2is and GLP-1RAs) and stabilize eGFR, along with continued regular monitoring.1 53 A proposed algorithm for the optimum management of CKD in T2D using albuminuria as a target is presented in figure 3. However, barriers to albuminuria testing exist, including confusion caused by the similarity of test names and results. For example, the typical range of results for UACR and urine albumin (UALB) is similar, but the UACR test measures albumin content relative to creatine (in units of mg/g) while the UALB test measures total albumin in urine (in units of mg/dL).54 Other barriers include a lack of understanding of testing guidelines and which tests to order; infrequent physician visits by patients or avoidance of treatment; and methodologic issues, such as urine collection instructions, requirement for specific urine cups, or an inadequate patient recall system when patients fail to provide a urine sample.54 55 The guideline-preferred method of screening is annual testing of albuminuria using the accurate and cost-effective UACR test (table 1).2 11 56
Methods for testing albuminuria as recommended by KDIGO and ADA guidelines2 11 54 57
Proposed algorithm for the optimum management of CKD in T2D using albuminuria as a target.2 65 †Exercise (strenuous exercise within 24 hours of sample collection), infection (septicemia or other conditions increasing vascular permeability, symptomatic urinary tract infection), fever, congestive heart failure, marked hyperglycemia, menstruation, upright posture (orthostatic proteinuria causing transient elevation in UACR), and marked hypertension may elevate UACR independent of kidney damage. A, albuminuria category; ACEi, ACE inhibitor; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; DPP-4i, dipeptidyl peptidase-4 inhibitor; eGFR, estimated glomerular filtration rate; G, GFR category; GLP-1RA, glucagon-like peptide-1 receptor agonist; RAS, renin–aldosterone system; SGLT2i, sodium-glucose co-transporter-2 inhibitor; T2D, type 2 diabetes; UACR, urine albumin-to-creatinine ratio.
Methods of measuring albuminuria are not standardized; however, KDIGO guidelines recommend standardization against a serum-based calibrant (CRM 470), with albumin measured using immunologic assays capable of specific and precise quantification at low concentrations and of producing quantitative results over the clinically relevant range.2 Alternate methods, such as a 24-hour UALB test, are available; however, this test is more burdensome (table 1).2 11 The use of urine dipsticks offers a convenient, cost-effective method of measuring albuminuria alone (table 1).2 11 However, the use of urine dipsticks is susceptible to false results with poor sensitivity for UACR ≥30 mg/g detection (~62%; 95% CI 50.9 to 72.0).2 57 58 Combining urine dipstick measurements for albumin with simultaneous measurement of creatinine could ensure a higher level of accuracy as this will correct for variations in albumin concentration that is highly dependent on hydration status.11 In addition, assays that measure total protein, such as colorimetric protein assays, may be used.2 11 However, it is important to note that UALB measurements provide a more specific and sensitive measure of changes in glomerular permeability than urinary total protein, and they enable early detection of low concentrations of albumin as a marker of kidney damage and the progression of kidney disease (table 1).2 Therefore, as kidney function declines, it is recommended that testing be carried out more frequently,2 with repeat testing of albuminuria in addition to eGFR measurements recommended to assess disease progression or response to treatment (figure 3).2
Discussion and conclusions
Albuminuria is a key marker of kidney damage and cardiorenal risk, and it is important to perform periodic tests for albuminuria in people with T2D, both to detect CKD and to monitor its progression. The CREDENCE (CKD and T2D population) and DAPA-CKD (CKD and mixed T2D and non-T2D population) trials of SGLT2is have shown that the presence of high albuminuria at baseline is predictive of elevated risk for future hard kidney outcomes;38 39 the Heart Outcomes Prevention Evaluation (HOPE) study identified albuminuria as a continuous risk factor for all-cause mortality and cardiovascular mortality;59 and in a meta-analysis of clinical trial data, lowering of albuminuria with SGLT2is correlated with delayed onset of hard kidney outcomes such as kidney replacement therapy.60 61 An increased focus on routine systematic testing for albuminuria in clinical practice is important to address the current suboptimal rates and overcome existing barriers for albuminuria testing. There has been a long-standing perception of no utility in screening for CKD. For decades, there were no interventions apart from ACE inhibitors and ARBs; often prescribed for hypertension or heart failure that could address a decline in kidney function. However, this is changing with an evolving landscape: SGLT2is are now indicated for CKD in people with T2D62 and positive outcomes are being reported for new agents that specifically target kidney damage (eg, non-steroidal MR antagonists). The KDIGO and American Diabetes Association guidelines now recommend that people with CKD and T2D, and an eGFR ≥30 mL/min/1.73 m2 would benefit from the use of SGLT2is, with or without metformin; GLP-1RAs recommended as a preferred additional therapy based on patient preferences, comorbidities, cost, and eGFR status1 because these therapies appear to reduce the risk of CKD progression, cardiovascular events, and hypoglycemia.11
The importance of education and awareness around albuminuria screening is underscored by the launch of a new public awareness initiative by the US Department of Health and Human Services, the National Kidney Foundation, and the American Society of Nephrology, which aim to provide education about the risks of CKD and promote the early detection, treatment and management of CKD to improve patient outcomes.63 Furthermore, a majority of CKD burden is concentrated in geographies with the lowest sociodemographic index,64 where early detection using cost-effective methodologies and early intervention could have a significant impact on reducing disease burden.
We strongly believe that the time is right for a far more aggressive and interventional approach to CKD in T2D that goes all the way from education and awareness to appropriate and regular screening and follow-up. This is particularly relevant in view of both the recent executive order on kidney diseases and the availability, for the first time, of novel therapies that could effectively prevent the progression of CKD.
Data availability statement
No data are available. The data supporting the findings of this study are not currently available.
Ethics statements
Patient consent for publication
Acknowledgments
Medical writing assistance was provided by Cindy Jenner, PhD, of Chameleon Communications International, and was funded by Bayer AG.
References
Footnotes
Contributors JBM and PR conceived the manuscript, approved the outline, edited outlines and all drafts and approved the final manuscript content. HH, PR-C, AC, TW, CW, LJ added expertise, edited the outline and drafts and approved the final draft. JBM is the guarantor of the work.
Funding This study was funded by Bayer.
Competing interests JBM reports non-financial support from Boehringer Ingelheim; personal fees from Bayer, Boehringer Ingelheim, MannKind, Novo Nordisk and Provention Bio; grants from Beta Bionics, Dexcom, Medtronic, and Novo Nordisk. PR-C is a consultant/advisor for Akebia, Bayer, Becton Dickinson, Cormedix, Humacyte, Medtronic, Vifor-Relypsa, and WL Gore. He is also the founder and CSO of Inovasc. AC is a consultant/advisor for Bayer. LJ reports receiving consulting and lecture fees from Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Merck, Novartis, Novo Nordisk, Roche, Sanofi, and Takeda; and research grants from Roche and Sanofi. HH reports personal fees from Alexion Pharma, AstraZeneca, Bayer, Boehringer Ingelheim, and Vifor Pharma, outside the submitted work. TW reports grants, personal fees and other from Kyowa Kirin; grants and personal fees from Astellas Pharma, Daiichi Sankyo, Kissei Pharmaceutical, Mitsubishi Tanabe Pharma, Sanofi, and Takeda Pharmaceutical; grants from Chugai Pharmaceutical and MSD; and personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly Japan, Kowa, Nippon, Ono Pharmaceutical, Sanwa Chemistry, and Taisho Pharma, outside the submitted work. CW reports consultancy and/or speaking fees from AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, GSK, Eli Lilly, MSD, and Mundipharma; and research grants from Boehringer Ingelheim and Sanofi Genzyme outside this body of work. PR reports lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Merck, and Novo Nordisk; consultancy fees from AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Mundipharma, and Novo Nordisk, with all fees given to Steno Diabetes Center Copenhagen; and equity interest in Novo Nordisk.
Provenance and peer review Not commissioned; externally peer reviewed.