Article Text
Abstract
Aims/hypothesis Dyslipidemia is an important modifiable risk factor and lipid-lowering treatment (LLT) is essential to reduce the risk of cardiovascular disease (CVD). Studies in type 2 diabetes indicate that low adherence to statin therapy is a barrier to reach full protective potential, and less is known in type 1 diabetes (T1D). The aim was to assess risk of CVD by adherence and nonpersistence to LLT in T1D.
Method A population-based study with a retrospective longitudinal design was conducted between 2006 and 2010, with follow-up until December 2013. In total, 6192 adult individuals with T1D, initiating LLT between 2006 and 2010, were included. Information on LLT, socioeconomic characteristics, comorbidities and cardiovascular events were collected. After 18 months, refill adherence was estimated by calculating medication possession ratio (MPR). Nonpersistence was defined as being without medicines on hand for at least 180 days. Individuals were thereafter followed until CVD, death or end of follow-up in December 2013. Cox regression analyses were performed to assess adherence level and nonpersistence of LLT as predictor of CVD. Analyses were adjusted for cardiovascular risk factors and socioeconomic status.
Results Mean MPR was 72%, 52% of the participants had an MPR above 80% and 27% discontinued LLT. There were 637 nonfatal and 58 fatal CVD events, mean follow-up 3.6 and 3.9 years, respectively. MPR above 80% was associated with reduced risk for nonfatal CVD compared with lower MPR, HR 0.78 (95% CI 0.65 to 0.93)). For fatal CVD, results indicated a negative effect of high adherence but the association did not reach statistical significance, HR 1.96 (0.96 to 4.01). Individuals discontinuing LLT had higher risk of nonfatal CVD, HR 1.43 (95% CI 1.18 to 1.73).
Conclusions/Interpretation In T1D, the risk for nonfatal CVD was lower among individuals with high adherence and higher among those discontinuing LLT within 18 months. It is important to evaluate and emphasize adherence to prescribed LLT at clinical visits to achieve treatment goals and reduce the risk of CVD.
- cardiac risk reduction
- lipid-lowering drugs/medication
- insulin-deficient Type 1 diabetes
- adherence to medications
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- cardiac risk reduction
- lipid-lowering drugs/medication
- insulin-deficient Type 1 diabetes
- adherence to medications
Significance of this study
What is already known about this subject?
Cardiovascular disease (CVD) is the leading cause of shorter life expectancy in type 1 diabetes.
Dyslipidemia is an important modifiable risk factor and lipid-lowering treatment is essential to reduce the risk of CVD.
Studies in general population and in type 2 diabetes indicate that low adherence to statin therapy is a barrier to reach full protective potential.
What are the new findings?
In individuals with type 1 diabetes initiating lipid-lowering therapy, high adherence to treatment was associated with 22% lower risk of nonfatal CVD.
Discontinuing the lipid-lowering therapy led to a 43% higher risk for nonfatal CVD.
How might these results change the focus of research or clinical practice?
It is important to evaluate and emphasize adherence to prescribed lipid-lowering therapy in clinical practice to achieve treatment goals and reduce the risk of CVD in individuals with type 1 diabetes.
Introduction
Despite positive trends in the latest decades, cardiovascular disease (CVD) is still the leading cause of shorter life expectancy in individuals with type 1 diabetes.1–3 Effects of hyperglycemia and nephropathy and also traditional risk factors such as smoking, hypertension, obesity and dyslipidemia are considered important for the excess of cardiovascular morbidity and mortality seen in type 1 diabetes.4 5
Lipid-lowering treatment (LLT) with HMG-CoA reductase inhibitors (statins) has in a multitude of studies proved to prevent major cardiovascular events and death, in the general population as well as in patients with diabetes.6 7 Cholesterol Treatment Trialists’ Collaborators meta-analysis of statins in 18 686 subjects with diabetes, the majority having type 2 diabetes, demonstrated a 21% reduction in major cardiovascular events for each 1.0 mmol/L (38.7 mg/dL) reduction in low-density lipoprotein (LDL) cholesterol.8 In an observational study from the Swedish National Diabetes Register (NDR), we could show a risk reduction of 22%–44% for CVD and death also in individuals with type 1 diabetes on LLT in primary prevention.9
In 2003, the WHO launched a report on adherence to medication for chronic diseases and concluded that poor adherence have large clinical impacts on health outcomes and utilization of health services worldwide.10 Chowdury et al published a meta-analysis in 2013 and calculated that approximately 9% of CVD events in Europe could be attributed to poor adherence to cardiovascular medication alone.11 Due to poor adherence, the effects of lipid-lowering medication seen in the context of a randomized controlled trial may be reduced in a real world diabetes care.12 Studies in the general population have shown an incremental improvement in clinical outcomes with the higher the adherence and the longer the persistence to LLT.13 14 In studies on patients with diabetes, the majority with type 2 diabetes, nonadherence to LLT have been associated with an increased risk for CVD and death both in primary and secondary prevention.15 16 Less is known in type 1 diabetes.
The aim of this study was to assess refill adherence to LLT and nonpersistence (ie, discontinuation) of LLT in individuals with type 1 diabetes in relation to cardiovascular outcomes and death. We hypothesized that high adherence and persistence to LLT in individuals with type 1 diabetes would be associated with a lower risk of CVD.
Research design and methods
Study population
We included individuals, 18 years or older, with type 1 diabetes in the Swedish NDR and who initiated use of LLT between 1 July 2006 and 31 December 2010. Type 1 diabetes was defined on the basis of epidemiological data: a diagnosis at the age of 30 years or younger and treatment with insulin alone. All patients were followed during the first 18 months on LLT until the first day of multidose dispensed medicines, death or migration, leading to censoring from the study. Individuals experiencing a nonfatal cardiovascular event during the 18-month exposure assessment period remained in the study. For the 18 months since initiation of LLT, we measured refill adherence to medication and time to discontinuation for each individual and thereafter followed them until a first cardiovascular event or until end of follow-up 31 December 2013. Events of all-cause death were followed until end of May 2015.
To identify novel users of LLT, we excluded individuals who filled a prescription of LLT within 365 days prior to inclusion, that is, prevalent users. See figure 1 for flowchart. Individuals were also excluded if they filled any prescriptions for lipid-lowering extemporaneous preparations, prescriptions lacking information of package size or monotherapy of bile acid sequestrants, the latter often prescribed for other indications than hyperlipidemia.17 We also excluded combination therapy users, unless bile sequestrants, if more than one substance or strength of the same substance were dispensed on the same date or if a previously filled substance or strength was filled once more within 45 days after the previous supply ended and another substance or strength was filled in between. Products containing multiple lipid-lowering substances in the same unit were considered monotherapy.
Flowchart for inclusion and exclusion criteria for the study population. NDR, National Diabetes Register.
Measurements of adherence and discontinuation
In the present study, we have investigated refill adherence and measured the medication possession ratio (MPR), that is, the proportion of days with medicines on hand according to filled prescriptions, during the first observation period of 18 months. The refill adherence is considered one of the most reliable objective measures of adherence in large patient groups with long-term treatments of chronic conditions.18 We used 18 months of exposure as recommended by a previous study of statins for the general Swedish population, giving us robust data on adherence with the possibility of at least six refills, since most prescriptions of long-term medication are commonly issued for 1 year treatment, with 3-month dispensation intervals.19 Using 18 months of exposure rendered enough follow-up time for evaluation of events related to adherence and discontinuation of therapy.
MPR was measured both as a continuous variable and as dichotomized with a cut-off value >80% defined as high adherence and ≤80% as low adherence. An 80% cutoff has commonly been used in other studies assessing adherence and is also the level above where the cardioprotective benefits of LLT, mainly statins, become obvious.20 21 We defined persistence as the length of continuous use from initiation to discontinuation of LLT. Patients who discontinued treatment within the first 18 months were considered nonpersistent to LLT. Discontinuation was defined as a gap of at least 180 days between two filled prescriptions for LLT, representing two refills within the Swedish reimbursement system. For these measurements, we used an algorithm defined in a previous study investigating adherence in individuals with type 2 diabetes.22
Registries used in the study
Clinical baseline characteristics were retrieved from the NDR. NDR was initiated in 1996 as a tool for local quality assurance and as a feedback tool in diabetes care.23 Each patient provides informed consent. Roughly 98% of all individuals age 18 and older with type 1 diabetes in Sweden are included in the register.
The Swedish Prescribed Drug Register (SPDR) provided information on age, sex, type of medicine, package size, date of dispensing and free text dosage instructions from the prescriber. The SPDR has since July 1, 2005 individualized its data on all prescriptions filled in Sweden and has been characterized in an earlier study.24
The unique Swedish personal identity number further made it possible to link register data from the Swedish National Patient Register, the Cause of Death Register (both administered by the Swedish National Board of Health and Welfare), as well as the Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA, administered by Statistics Sweden). The NPR has nationwide coverage since 1987 and includes mandatory information on all principal and secondary hospital discharge diagnoses classifying them by The International Classification Disease (ICD) system; we used ICD-10 with documented discharge diagnoses since 1997. Sensitivity and specificity for diagnoses of acute myocardial infarction, coronary heart disease, hospitalization for heart failure, atrial fibrillation and stroke have been validated.25 26 From LISA, we obtained data on socioeconomic status27 and from the Cause of Death Register date and cause of death.
The study was approved by the Regional Ethics Review Board at the University of Gothenburg, Gothenburg, Sweden.
Examinations at baseline
Clinical data from the NDR: Data were collected before index date up to 2 years prior to inclusion with last observation carried forward (LOCF). LDL-cholesterol values were calculated using Friedewald's formula if triglycerides<4.0 mmol/L.28 Microalbuminuria was defined as albumin/creatinine ratio 3–30 mg/mmol or U-albumin 20–200 µg/min or 20–300 mg/L and macroalbuminuria as albumin/creatinine ratio >30 mg/mmol or U-albumin >200 µg/min or >300 mg/L. Glomerular filtration rate was estimated with the MDRD (modification of diet in renal disease) equation.29 Smoking was coded as present if the patient was a current smoker. Physical activity was categorized into low or high level where low exercise meant to never exercise or to exercise less than 1 time per week while high meant exercise more than 1–2 times per week.
Socioeconomic status from LISA: Data on disposable individual income in hundred Swedish kronor (latest annual income, not adjusted for inflation), highest educational level, country of birth and marital status were retrieved from the register ±1 year from index date using the closest value. Education was stratified into compulsory school or lower (≤9 years), upper secondary school (10–12 years) and postsecondary (college/university). Immigrant status was defined as Swedish native or immigrant, depending on country of birth. Marital categories were single, married, divorced or widowed.
Comorbidities and events: Collected before baseline examination and during follow-up by linking data from the NDR to the Swedish National Patient Register and the Causes of Death Register. Information on prior comorbidities was retrieved before index back to 1997 with LOCF. The following comorbidities and events were assessed: myocardial infarction (ICD-10 code I21), unstable angina (I20.0), percutaneous coronary intervention (PCI) and/or coronary artery bypass grafting (CABG), coronary heart disease (I20–I25), stroke defined as cerebral infarction, intracerebral hemorrhage or unspecified stroke (I61, I63, I64), peripheral vascular disease (PVD) defined as peripheral atherosclerosis in the arteries of the extremities (I70.2, I73.9, I79.2) or diabetes mellitus with complications in the peripheral arteries (E10.5, E11.5, E14.5), endovascular intervention in the peripheral arteries and/or peripheral artery bypass grafting, atrial fibrillation (I48), history of congestive heart failure (I50) and any cancer (C00–C97). For events, we evaluated total mortality and nonfatal or fatal CVD and a composite of nonfatal and fatal CVD. In the cox regression analysis a CVD event was a composite of myocardial infarction, unstable angina, PCI, CABG, stroke, PVD, endovascular interventions and/or peripheral artery by-pass grafting. In the spline analysis a nonfatal CVD was a composite of unstable angina, myocardial infarction, stroke and PVD.
Missing data: For clinical characteristics there were 21% missing data, varying between variables, from no missing on gender to 17% missing on HbA1c and 33% missing for information on physical activity. For socioeconomic variables, only 0.6% were missing information on education and 0.1% on marital status and income respectively. There were no missing data on comorbidities.
Statistical analyses
The observed data are described using standard descriptive statistics such as mean, SD, counts and percentages. A Cox proportional hazard regression analysis with HRs with 95% CI was performed to analyze MPR as predictor of nonfatal and fatal CVD and all-cause death, comparing the group with MPR>80% to the group with MPR≤80% and also comparing discontinuers versus continuers of LLT in relation to risk for a cardiovascular event or death. We also performed a sensitivity analysis with a Cox regression analysis as above but excluding the patients having a nonfatal CVD event during the exposure assessment period. The Cox regression analyses were adjusted for traditional risk factors, comorbidities and socioeconomic status as presented in table 1. Missing data were handled by means of multiple imputation. We used multiple imputation by chained equations algorithm imputing 10 complete datasets. Separate analyses were run on each imputed data set and the results were combined using Rubin’s rules.
Number of events and adjusted HRs (95% CI) for MPR as a continuous measure (MPR 18 months) per one unit increase and for high refill adherence (MPR>80%) with MPR>80% compared with MPR≤80% and discontinuers* compared with continuers after 18 months
Baseline characteristics and adherence data for the study population; in all, in individuals with MPR≤80 and >80% and in discontinuers and continuers of LLT at index and 18 months after initiation of LLT
The impact of MPR was further investigated by fitting a Cox regression model where the effect of MPR as a continuous measure was modeled by a smoothing spline with 7 df to one of the imputed data sets, adjusted for age, sex and previous CVD. All hypothesis tests were evaluated using a 5% significance level without any adjustment for multiple comparisons. The analysis was performed using SAS 9.4 and R 3.4.3.
Results
Table 2 presents baseline characteristics and adherence data for all 6192 participants by high and low adherence and also discontinuers and continuers as a measure of persistence. In summary, mean age was 45 years, diabetes duration 29 years and 58% were of male gender. Forty-three per cent had antihypertensive medication. Previous CVD was present in 9% of the participants. Ninety-three per cent were born in Sweden. Forty-five per cent were married and 12% divorced. Seventeen per cent had an education of 9 years or less, 29% had gone to college or university.
The mean MPR over 18 months was 72%±28% (median 83%), 52% of the participants had an MPR>80% (median 97%) and 27% discontinued with LLT during the 18-month exposure assessment period. In the group with high adherence, the frequency of individuals with previous CVD and with concurrent medication (antihypertensives and anticoagulants) was higher. Smokers were less adherent than nonsmokers. The majority of the individuals, 99%, were on treatment with statins, simvastatin being the most common at 94.4%, followed by atorvastatin at 3.4% (see online supplementary table 1).
Supplemental material
Table 1 shows number of events and fully adjusted HRs. There was a total of 637 nonfatal CVD events, 58 fatal CVD events and 302 deaths for other causes during mean follow-up 3.6 years for nonfatal events and 3.9 years for fatal events. Patients with a high adherence (MPR >80%) had a 22% lower risk of nonfatal CVD compared with patients with MPR ≤80%. Patients discontinuing LLT had a 43% higher risk of nonfatal CVD. For those with an MPR>80%, an association to increased risk of fatal CVD HR 1.96 (0.96 to 4.01) was found, but it did not reach statistical significance (p=0.06). For the composite of nonfatal and fatal CVD, there was a 21% significantly reduced risk with high adherence to LLT. There was no association between adherence or discontinuation and total death.
In the extra analysis excluding the individuals that had a nonfatal cardiovascular event during the exposure assessment period, the point estimate for fatal CVD changed only marginally (MPR 18 months>80% 1.98 (0.86 to 4.52), p=0.11).
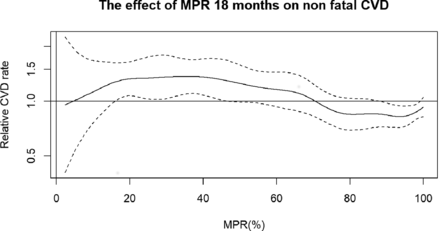
The relative rate of nonfatal CVD (95% CI) in relation to MPR as a continuous measure is illustrated in figure 2. The cubic spline shows higher CVD risk with lower MPR with the highest risk in those with an MPR from 20% to 40% and the lowest risk in those with an MPR above 80%.
The effect of MPR calculated over 18 months on risk of nonfatal CVD. Relative CVD rate with 95% CIs adjusted for age, sex and previous CVD (including coronary artery bypass grafting, percutaneous coronary interventions and endovascular grafting). Nonfatal CVD: composite of unstable angina, myocardial infarction, stroke, peripheral vascular disease, coronary artery bypass grafting, percutaneous coronary intervention and endovascular grafting. CVD, cardiovascular disease; MPR, medication possession ratio.
Discussion
This observational nationwide study in 6192 individuals with type 1 diabetes was designed to assess refill adherence to lipid-lowering therapy (LLT) in a real-life setting and also to evaluate adherence in relation to the risk of a cardiovascular event, fatal or nonfatal and all-cause death. Our results show a significant effect of high refill adherence with a 22% reduced risk of developing a nonfatal cardiovascular event for the individuals with an MPR>80% compared with those with a an MPR≤80%. In individuals discontinuing therapy during 18 months of exposure, there was a significantly increased risk of 43% for a nonfatal cardiovascular event. For the composite of nonfatal and fatal CVD, we observed a 21% significantly reduced risk with high adherence to LLT.
Adherence to medication is important to gain full potential of LLT and reach the results of primary and secondary prevention with LLT seen in randomized controlled trials. In a systematic review of 19 journal articles from 1999 to 2009 on adherence to statins and its impact on outcome, Simpson et al concluded that high levels of adherence were associated with significant reductions in a range of outcomes such as fatal and nonfatal CVD as well as all-cause death and hospitalizations.13 Deshpande et al could confirm these findings in a more recent review published 2017, assessing 84 real-world studies on adherence and persistence from 2006 to 2016.21
Many studies show, unlike our study, a significant effect of high adherence on cardiovascular death and all-cause death.30 31 In our study, the analysis for fatal CVD showed an association with high adherence to LLT, however nonsignificant. The number of fatal cardiovascular events were few and when analyzing the composite of nonfatal and fatal CVD, the association was significantly positive for high adherence to LLT. Since individuals experiencing a CVD event during the exposure assessment period remained in the study, both adherence and outcome could be affected. One could expect a higher adherence to LLT directly adjacent to an event and also a higher risk for a new event in the near future. However, the sensitivity analysis of those with no event during the exposure assessment period showed only marginal effects on the estimates. Hence, the unexpected observation for CVD death is likely to be biased and due to residual confounding, unmeasured or inherited by the study design.
The evidence for the efficacy of LLT in prevention of CVD in diabetes is undisputed.8 32 33 Poor adherence to cardiovascular medication alone account for approximately 9% of CVD events in the general population.11 In our study, only 52% of the participants had an MPR above 80% after 18 months. This level of adherence is consistent with the adherence measures in a review by Cramer et al.20 They reviewed studies published between 2000 and 2005, on adherence to treatment for diabetes, hypertension and dyslipidemia, revealing that only 51% of patients treated with LLT were having medication on hand for more than 80% of their days on therapy after 12 months.20 Adherence studies in other populations than ours have shown that older age, concurrent medication, presence of CVD and other comorbidities such as type 2 diabetes, tend to increase adherence to statins, the most prescribed of LLT.34 35 In our fairly young cohort, 9% had a history of CVD and 43% were treated with antihypertensive medication, which is reflected in the level of adherence where those with prior CVD and with other medications than insulin more often were among those with high adherence. In a study on adherence to statin guidelines in patients with type 1 diabetes and with over 50 years of diabetes duration, 72% of the patients self-reported adherence to statins, but fewer in primary than in secondary intervention, 68.5 vs 84.4% respectively.36 In the general population and in type 2 diabetes, adherence to statins tends to deteriorate over time and has been reported to be below 25% in high-risk patients after 5 years of treatment.14 37 38 In our study of individuals with type 1 diabetes, 27% had discontinued LLT after 18 months. Early diminishing use and discontinuation of LLT is unfortunate since high levels of adherence and longer duration of persistence with statins are associated with incremental improvement in clinical outcomes in patients at risk for cardiovascular events.13 14 39
One reason why patients do not stay on treatment could be unwarranted side effects. In a large survey by Wei et al, perceived muscle pain was the far most common reason for nonpersistence accounting for 60% of discontinuation.40 In randomized controlled trials, serious adverse events from treatment with statins are sparse though, also when it comes to muscle-related problems, and when experienced side-effects from statins have been tested against placebo, there was no longer a significant difference between placebo and active substance.41–43 Claims of nonserious but symptomatic side effects can accordingly be a hindrance to treatment adherence and hence a cause for insufficient cardiovascular prevention. Healthcare professionals are important coactors in this. In a recent study from Italy, reasons for discontinuation of statins were assessed in 655 patients with suspected statin-induced adverse reactions and revealed that over 80% of statin discontinuations were due to adverse reactions classified as not serious, concluding that healthcare professionals’ had a substantial impact on persistence to LLT, by discontinuing medication for not valid reasons.44
Perceived lack of efficacy can also be a reason for low adherence and discontinuation of statin therapy.40 One way to counteract perceptions like this could be to follow the recently published guideline on Cardiovascular Disease and Risk Management, from American Diabetes Association and the guideline on Management of Cholesterol from American College of Cardiology/American Heart Association, updated in January 2018 and November 2018, respectively, and regularly assess LDL-cholesterol after initiation of statin therapy in order to increase adherence.45 46 Some guidelines are now suggesting adding more pharmacological treatment to statins in order to reach LDL-cholesterol treatment targets in patients at high risk for CVD. If adherence to statin therapy was regularly scrutinized in clinical practice, treatment targets might be reached in more patients with statin treatment alone.
Strengths and limitations
There are several strengths of this study: first, the large sample of individuals with type 1 diabetes being novel users of LLT; second, the prospective approach and capacity to reflect usage in a real-world setting; third, our register-based study provides detailed data on cause of death, socioeconomic status, major comorbidities as well as risk factors including laboratory measures.
There are also limitations. In an observational study, there is always a possibility for residual confounding due to unmeasured variables, for example, hereditary factors and comorbidities such as psychiatric disease, which could influence both adherence and outcome.47 We may also have overestimated adherence as we do not know that all filled medications are consumed as prescribed, which would attenuate the association between adherence and CVD. Another bias to consider is the healthy adherer bias. High adherence could be a marker of healthy behavior and therefore a lower risk of a CVD event, even if we adjust for body mass index, physical activity and smoking as a proxy. Taking this into consideration, our results on lower CVD risk with high adherence could be overinterpreted. On the other hand, we excluded patients that could be of a certain fragility. Only patients who survived the exposure period were eligible for inclusion and we also excluded individuals with multidose dispensed medications and individuals with combination therapy, who are often older and with more concurrent diseases and with a higher risk for a cardiovascular event. These exclusions could lead to a selection bias with younger and somewhat healthier participants than in a real-life setting, hence underestimating the cardiovascular risk in our population and attenuating the impact of adherence.
Conclusion
This nationwide study among individuals with type 1 diabetes individuals and novel users of LLT shows that high refill adherence to LLT was associated with 22% lower risk for nonfatal CVD. Individuals discontinuing LLT within 18 months had a 43% higher risk of nonfatal CVD. It is important to evaluate and emphasize adherence to prescribed LLT at clinical visits in order to reach full potential of LLT and so reduce the risk of CVD in individuals with type 1 diabetes.
Acknowledgments
The authors thank the regional National Diabetes Register (NDR) coordinators, as well as contributing nurses, physicians and patients.
References
Footnotes
Contributors Study concept and design: CH, SAK, A-MS, KAS, BE and KE-O. Statistical analyses: MM and SF. Interpretation of data: CH, SAK, KAS, A-MS, BE, KE-O, SF and SG. Drafting of manuscript: CH and KE-O. Critical revision and completion of manuscript by all authors. The manuscript has been read and approved by all coauthors.
Funding The study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG-698991 and ALFGBG-430711) and from the Gothenburg Society of Medicine. The Swedish Diabetes Association and the Swedish Society of Diabetology support the NDR. The Swedish Association of Local Authorities and Regions funds the NDR.
Competing interests KAS is employed by AstraZeneca. However, the views expressed in this study are her own and not those of AstraZeneca. SG has received personal fees (lecture fees and research grants) from AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk and Sanofi. KE-O has received personal lecture fees from Eli Lilly and Abbott. BE has received personal fees for lectures and serving on advisory boards from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Mundipharma, Navamedic, Novo Nordisk and RLS Global and research grants from Sanofi. CH has received personal lecture fees from Eli Lilly.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement No data are available. The data that support the findings of this study are not publicly available. The study presented here have been subject to an application to an ethical board and approved for publication related to the specific aim of our research project. With reference to the European General Data Protection Regulation (GDPR), the data are personal data and thereby protected by secrecy. Study definitions and descriptive statistics are available on request.