Article Text
Abstract
Introduction Previous studies have shown disruption of glycometabolic control and new diabetes mellitus (DM) diagnosis among patients with COVID-19. It is still unclear how the association of COVID-19 and new-onset DM may be modified by disease severity or vary over time, during acute and post-acute phases.
Research design and methods In this retrospective matched cohort study, 157 936 patients with COVID-19 (aged ≥25 years, diagnosis date between March 01, 2020 and August 31, 2021) were compared with individuals without COVID-19, separately for non-hospitalized, hospitalized, and severe hospitalized patients. Stratified Cox proportional hazards models, with changing baseline time (starting at the date of COVID-19 diagnosis, and at 1, 2, 3, and 4 months afterwards), were used to evaluate the occurrence of new DM in relation to COVID-19 infection in different time frames—from each landmark date until end of study.
Results During mean follow-up time of 10.9 months, there were 1145 (0.72%) new diagnoses of DM compared with 1013 (0.64%) in the individuals without COVID-19 (p=0.004). Non-hospitalized patients with COVID-19 were not at higher risk of new DM neither during the acute phase nor afterward. Hospitalized patients with COVID-19 had a higher risk of developing DM, with the highest risk among severe hospitalized patients. This risk among hospitalized patients was highest in the acute phase (HR 2.47 (95% CI 1.86 to 3.29)), attenuated over time, but remained significant at 4-month landmark analysis (HR 1.60 (95% CI 1.12 to 2.29)).
Conclusions Acute and post-acute COVID-19 were associated with new DM only among hospitalized patients, with the highest risk among those hospitalized with severe disease. Those patients should be followed and monitored post-discharge for new DM. Patients who were not hospitalized did not have higher risk of new-onset DM.
- COVID-19
- Diabetes Mellitus, Type 2
- Longitudinal Studies
- Electronic Health Records
Data availability statement
No data are available. The data are not publicly available due to privacy restrictions.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Previous studies have shown disruption of glycometabolic control and new diabetes mellitus (DM) diagnosis among patients with COVID-19, mostly among hospitalized patients during the acute phase of the disease.
Few studies have demonstrated an excess risk of new DM also among non-hospitalized patients but assessed the association of COVID-19 with new DM in the context of long COVID-19, among old men, or compared with individuals with acute upper respiratory tract infections. More studies are needed on incidence of DM among patients with COVID-19 with mild illness managed in the community, as well as on the association of COVID-19 with DM incidence in different time frames in relation to COVID-19 diagnosis.
WHAT THIS STUDY ADDS
Compared with individuals without COVID-19, non-hospitalized patients were not at higher of new DM.
Hospitalized patients had a higher risk of new DM during the acute and post-acute phase, with the highest risk among severe hospitalized patients.
This risk among hospitalized patients was highest in the acute phase, attenuated over time, but remained significant during the post-acute period.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Patients who were hospitalized due to COVID-19 should be followed and monitored for new DM.
Introduction
Diabetes mellitus (DM) is recognized as a common comorbidity of COVID-19 and is a risk factor for greater severity of the infection and additional complications.1–4 Alteration in the glycemic balance and appearance of new-onset DM in patients with COVID-19 have been reported.5 6 Suggestive mechanisms have been described,7–14 focused mainly on short-term early clinical implications during acute infection. Previous studies have predominantly focused on disruption of glycometabolic control and new DM diagnosis among hospitalized patients.15–24 There are limited data on patients with milder illness managed in the community.25 26 In addition, it is unclear whether the excess risk for new DM changes over time after the period of acute infection has resolved. The objective of this study was to evaluate the time-varying association between COVID-19 infection and the risk of developing new DM as compared with matched individuals who were not infected by COVID-19, stratified by the severity of the disease. Specifically, this study aimed to assess the association of COVID-19 infection with new-onset DM at five ‘landmark’ times (date of COVID-19 diagnosis, and 1, 2, 3, and 4 months after diagnosis) separately for non-hospitalized patients, hospitalized individuals, and those hospitalized with severe COVID-19.
Methods
Study setting
Clalit Health Services (Clalit) is the largest healthcare organization (insurer and provider) in Israel where health insurance is universal and mandatory for all citizens. Clalit provides primary, secondary, and tertiary care to 4.8 million individuals (52% of the population) and owns and operates a network of hospitals which account for 30% of all general hospital beds in the country. Clalit’s information systems are fully digitized and feed into a central data warehouse including both administrative and clinical data.
Study design
This retrospective, population-based cohort study included all Clalit members who were diagnosed with COVID-19 between March 01, 2020 and August 31, 2021 and matched individuals who were not infected by COVID-19. The date of COVID-19 diagnosis was considered as the index date. Baseline variables including sociodemographic clinical and treatment characteristics were defined based on the most recently available data prior to index date.
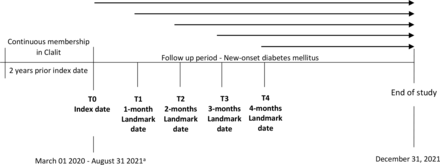
Five landmark times were defined, at index date (T0) and at 1 (T1), 2 (T2), 3 (T3), and 4 (T4) months post-index date. At each landmark date, individuals with COVID-19 and their matched controls were followed until an occurrence of one of the following events: new documentation of DM, occurrence of COVID-19, pregnancy, corticosteroid use in the outpatient setting, disruption in Clalit membership, all-cause mortality or end of study (December 31, 2021). This landmark design enabled to assess the association of COVID-19 with incident DM during the acute and the post-acute phase in varying time frames, as well as to reduce the possibility of reverse causality and surveillance bias (figure 1).
Study design. aPersonal index date was defined for each participant based on date diagnosis of COVID-19, with a maximum potential follow-up period of 22 months (from March 01, 2020 to December 31, 2021).
Study population
All Clalit members aged ≥25 years with documented positive PCR test for COVID-19 during March 01, 2020–August 31, 2021 and with continuous membership at Clalit during the 2 years prior to the index date were included in the study. The minimum inclusion age of 25 years was determined to avoid a lack of data during the period of mandatory service in the Israel Defense Forces. For each individual diagnosed with COVID-19, one individual who was not infected by COVID-19 (before index date) was matched based on age group (stratified by 5-year intervals), sex and primary care clinic/neighborhood characteristic which represents different ethnic and religious groups in Israel (Arabs, Orthodox Jewish, traditional/secular Jewish). Matching for primary care clinic/neighborhood characteristics was performed due to statistically significant differences in DM27 28 and COVID-1929 incidence between the varied communities living in different neighborhoods in Israel. Individuals were excluded if they met one or more of the following criteria: documentation of DM before the index date; positive antibody test for COVID-19 during the study period without prior vaccination or positive PCR test (to remove individuals for whom there was probably an undocumented COVID-19 event); pregnancy at index date; corticosteroid use during the 3 months prior to index date (based on Anatomical Therapeutic Chemical (ATC) code H02, at least one filled prescription in the outpatient setting); long-term care facility housing (home nursing or psychiatric geriatric or rehabilitative hospital); and home confinement for medical reasons. The last two exclusion criteria were set due to absence of continuous medical reporting on the long-term care facility residents, and accordingly, substantial missing information in Clalit data warehouse related to this subpopulation. Non-COVID-19 matches met the same inclusion and exclusion criteria as the patients with COVID-19 except the positive PCR test at index date. Potential matches were selected irrespective of their future COVID-19 status; therefore, there were patients in the matched non-COVID-19 group who were diagnosed with COVID-19 during the follow-up period time. Those individuals and their matched pair were censored at the time of their COVID-19 diagnosis. Patients with COVID-19 for whom suitable matches were not found were excluded from the analysis (figure 2). To evaluate whether the association of COVID-19 with new-onset DM varies by disease severity, three subgroups were defined including non-hospitalized patients, hospitalized patients, and those hospitalized with severe COVID-19.
Selection of study population. *Those individuals and their matched pair were included in the analysis but were censored at the time of their COVID-19 diagnosis. T2DM, type 2 diabetes mellitus.
Baseline measurements
Incident COVID-19 was assessed based on documentation of a positive PCR test for SARS-CoV-2 in the hospitals or community laboratory records. Information regarding laboratory-confirmed COVID-19 infections was also received from the Israeli Ministry of Health which established and maintains a national database with mandatory daily reporting of PCR results and disease status from all hospitals in Israel. Three levels of COVID-19 severity were defined including not hospitalized, hospitalized, and severe hospitalized individuals (SpO2 <94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen <300 mm Hg, a respiratory rate >30 breaths/min, or lung infiltrates >50%).30–33 DM as of index date was determined based on documentation of one or more of the following criteria in hospitals or community records: International Classification of Diseases Ninth Revision (ICD-9) code 250xx, HbA1c ≥6.5%, and glucose-lowering medication use (ATC classification system code A10), defined as ≥1 dispensed prescription any time prior to index date. Individuals with documented fasting glucose levels of ≥126 mg/dL in the community records only and individuals listed with DM in the Clalit chronic disease registry were also considered as patients with DM at index date. The demographic variables assessed at the index date included age, sex, primary care clinic location (according to the main ethnic and religious group living in the clinic area), and socioeconomic status (low, medium, or high based on the primary care clinic level). Smoking status (non-smoker, former, or current) was evaluated based on last documentation in the community records as reported by the patient. Body mass index (BMI) (kg/m2), systolic and diastolic blood pressures (mm Hg), and laboratory data were gathered from the community clinic records based on last documented level prior to index date. Laboratory data included fasting plasma glucose concentration (mg/dL), HbA1c concentration (%), total cholesterol (mg/dL), high-density lipoprotein (HDL) cholesterol (mg/dL), low-density lipoprotein (LDL) cholesterol (mg/dL), and triglycerides (mg/dL). Medical diagnoses were defined based on ICD-9 codes documented in the hospital or the community clinic records anytime prior to the index date (online supplemental etable 1) and included pre-diabetes, hypertension, cardiovascular disease (CVD), congestive heart failure, hyperlipidemia, chronic kidney disease, and polycystic ovary syndrome (PCOS)/polycystic ovarian disease. CVD was defined by having one or more of the following diagnoses: myocardial infarction, unstable angina pectoris, stable angina pectoris, ischemic heart disease, ischemic stroke (cerebrovascular accident), coronary artery bypass graft, and percutaneous transluminal coronary angioplasty. Pharmaceutical treatment was defined according to the ATC classification system based on at least one filled prescription during the 2 years prior to index date. Medications included beta-blocking agents (ATC code C07), calcium channel blockers (ATC code C08), agents acting on the renin–angiotensin system (RAS) (ATC code C09), and lipid-modifying agents (ATC code C10).
Supplemental material
Primary outcome
New diagnosis of DM (yes/no) during the follow-up period was defined based on fulfillment of one of the following criteria: (a) new documentation of DM diagnosis—ICD-9 code 250xx in hospitals and community records; (b) HbA1c ≥6.5% based on hospital and community records; (c) at least two documentations of fasting glucose levels of 126 mg/dL or higher based on community records only; and (d) dispensed prescription of glucose-lowering medication (ATC code A10) based on hospital and community records. Since metformin or liraglutide is also used for treating conditions other than diabetes, individuals who started only those medications during the follow-up period without any other criteria were not considered as new DM cases. The earliest of these criteria was defined as the date of diagnosis.
Statistical analysis
Main characteristics are presented for COVID-19 and non-COVID-19 as means with SDs or medians with IQRs for quantitative variables that were normally or non-normally distributed, respectively. Categorical variables are presented by percentages. The association of COVID-19 with DM was evaluated by Kaplan-Meier non-parametric method and the log-rank test was used to compare the survival curves of COVID-19 and non-COVID-19 groups. HR and 95% CI for the association between COVID-19 and occurrence of new DM were assessed by stratified Cox proportional hazards model using 1:1 matched pair. Models were adjusted for age (years), socioeconomic status, pre-diabetes diagnosis of hypertension, diagnosis of dyslipidemia, diagnosis of PCOS, smoking status, BMI (kg/m2), glucose concentration (mg/dL), LDL cholesterol (mg/dL), HDL cholesterol (mg/dL), triglycerides (mg/dL), and lipid-modifying agents. To assess the possibility of period effect due to change in the therapy and severity of the infection over time, the follow-up was divided into three periods (January 20–August 31, 2020, September 01, 2020–March 31, 2021, and April 01, 2021–August 31, 2021), and the significance of the main effect of the period and the interaction period×COVID-19 were evaluated. To evaluate the possibility of surveillance bias,34 that is, capturing diagnosis of diabetes more in patients with COVID-19 than among non-COVID-19 matches, the percentage of individuals who had any interaction with the primary care clinics (that is, virtual visits, in-person visits, and blood glucose tests) was measured and compared between the two groups.
Individuals were censored from the analysis based on new diagnosis of DM, all-cause mortality, disruption in Clalit membership, COVID-19 diagnosis among controls, pregnancy, corticosteroid use in the outpatient setting, or end of study (December 31, 2021), whichever came first. The association of COVID-19 with DM was also evaluated for each of the COVID-19 severity groups. Five landmark models35 were performed including baseline and 1, 2, 3 and 4 months after COVID-19 diagnosis, evaluating the occurrence of new DM in different time frames in relation to COVID-19 infection with continued follow-up until the end of the study (December 31, 2021). Analyses at landmark 1, 2, 3 and 4 excluded patients with DM diagnosed from index date until the landmark date. Finally, five HRs were calculated, each of them representing the average HR for the specified period (from landmark date until the end of the study), and a time-varying association was assessed based on changes between these HRs. All statistical analyses were performed using R Statistical Software V.4.1.1. R package MICE V.3.1336 was used to impute missing baseline characteristics of the study population.
Results
Between March 01, 2020 and August 31, 2021, there were 269 586 individuals aged ≥25 years with documentation of a COVID-19 diagnosis and with continuous membership in Clalit during the 2 years prior to diagnosis date. Of these, 67 559 were excluded due to type 2 DM (T2DM)-associated criteria prior to index date, exposure to corticosteroids during the 3 months prior to index date, positive antibody test for COVID-19 prior to index date without prior vaccination, pregnancy at COVID-19 diagnosis test date, long-term care facility resident, or confined to home for medical reasons. Also excluded were another 27 individuals who did not meet the matching requirement of age, gender, and sector. Additionally, 44 064 patients with COVID-19 were selected as non-COVID-19 matches prior to their diagnosis date and were censored at the time of later COVID-19 diagnosis. The final study population consisted of 157 936 pairs at index date, 150 226 pairs at 1-month landmark date, 146 823 pairs at 2-month landmark date, 143 740 pairs at 3-month landmark date, and 140 982 pairs at 4-month landmark date. The interaction with the primary healthcare system during the follow-up period was higher among patients with COVID-19 (98.2%) compared with controls without COVID-19 (57.5%). This difference reduced throughout the landmark times with 31.0% and 26.4% among patients with COVID-19 and controls without COVID-19, respectively.
Main characteristics of the study population and amount of missing data for each variable at index date (<10% missingness in total) are described in table 1.
Baseline characteristics of COVID-19-positive patients and matched controls without COVID-19*
During a mean follow-up period of 10.9 months, there were 1145 (0.72%) and 1013 (0.64%) new cases of DM among the patients with COVID-19 and the controls without COVID-19, respectively.
Kaplan-Meier non-parametric models demonstrated differences in the survival distribution between patients with COVID-19 and controls without COVID-19 among the entire population as well as among hospitalized and severe hospitalized patients with COVID-19 (Plog-rank test <0.001 for all analyses). No difference in the survival distribution was found among non-hospitalized patients and their controls without COVID-19 (Plog-rank test=0.2) (figure 3).
Kaplan-Meier estimated new-onset diabetes mellitus for COVID-19-positive patients and matched individuals without COVID-19.
Figure 4 shows the association of COVID-19 with new-onset DM by using stratified Cox proportional hazards models. Unadjusted and adjusted (online supplemental etable 2 and figure 4) models starting at index date demonstrated a statistically significant association between COVID-19 and the risk of developing new DM as compared with matched individuals who were not infected by COVID-19 (unadjusted HR 1.16 (95% CI 1.07 to 1.27), adjusted HR 1.19 (1.09 to 1.29)). The adjusted association was attenuated when 1-month landmark analysis was applied (adjusted HR 1.12 (95% CI 1.02 to 1.23)) and preserved throughout the subsequent landmark models. When stratified by COVID-19 severity, no association was demonstrated for non-hospitalized patients neither at index date nor after the acute phase. Among hospitalized individuals, adjusted hazards models starting at index date demonstrated statistically significant associations between COVID-19 status and incidence of DM with higher risk among patients with COVID-19 compared with controls without COVID-19 (HR 2.47 (95% CI 1.86 to 3.29)). The highest risk of new-onset DM at index date was demonstrated among severe hospitalized patients (HR 3.33 (95% CI 1.94 to 5.72)). When landmark models were applied, the excess risk among hospitalized patients with COVID-19 compared with controls without COVID-19 was still manifested but attenuated over time (HRLM1 1.85 (95% CI: 1.36 to 2.53); HRLM2 1.73 (95% CI 1.24 to 2.43); HRLM3 1.68 (95% CI 1.19 to 2.37); and HRLM4 1.60 (95% CI 1.12 to 2.29)). Similarly, the excess risk among severe hospitalized individuals with COVID-19 compared with controls without COVID-19 decreased throughout the landmark models with no statistically significant association 3 months after diagnosis up to the end of study (HRLM1 2.18 (95% CI: 1.16 to 4.10); HRLM2 2.02 (95% CI 1.02 to 4.00); HRLM3 1.95 (95% CI 0.95 to 3.97); and HRLM4 1.80 (95% CI 0.88 to 3.88)). No statistically significant difference was observed for the main effect of period (p=0.56) and for the interaction effect of period×COVID-19 (p=0.28).
Supplemental material
Time-varying association of COVID-19 with new-onset diabetes mellitus (DM). aVariables included: DM (no/yes) (dependent variable), age (years), socioeconomic status (low, medium, high), pre-diabetes (no/yes: diagnosis of pre-diabetes or glucose concentration between 100 and 125 mg/dL or HbA1c between 5.7 and 6.4), diagnosis of hypertension (no/yes), diagnosis of dyslipidemia (no/yes), diagnosis of polycystic ovary syndrome (no/yes), smoking status (current, former/never), BMI (kg/m2), glucose concentration (mg/ dL), LDL cholesterol (mg/dL), HDL cholesterol (mg/dL), triglycerides (mg/dL), and lipid-modifying agents (no/yes). BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PY, person-years.
Discussion
In this retrospective, population-based cohort study, non-hospitalized patients with COVID-19 were not at higher risk of new DM either at the acute phase nor afterward compared with matching patients without COVID-19. Patients who were hospitalized due to COVID-19 had a higher risk of developing new DM compared with matching controls without COVID-19. This association was higher in the acute phase and attenuated over time. A statistically significant association was still observed among hospitalized patients with COVID-19 when 4-month landmark analysis was applied, suggesting excess risk for new DM among hospitalized patients during the post-acute phase. The association at the acute and the post-acute phases varied by disease severity with highest risks among individuals hospitalized with severe illness.
The observed association of COVID-19 with new DM during the acute phase may reflect the potential effect of SARS-CoV-2 on DM incidence. Several theories for this association between COVID-19 and hyperglycemia were described implicating mainly the effects of COVID-19 on ACE2 receptor in the different parts of the pancreas, the cytolytic effects on pancreatic beta-cells, and activation of the RAS. The transdifferentiating effect of beta-cell and increasing glucagon as an explanation for the increase in glucose level was also suggested.7 8 14 These theories relate mainly to the immediate effect of the SARS-CoV-2 suggesting mechanisms for new DM during the acute phase. Other explanations should also be considered.17 These include reverse causality, misdiagnosis of DM due to acute illness-hyperglycemia37 or misdiagnosis of DM due to use of corticosteroids during the acute phase among hospitalized patients.38 Using landmark analysis at different baseline times after the acute phase enabled us to relate the observed excess risk of DM to the exposure to COVID-19 more than to acute illness-hyperglycemia or use of corticosteroids during the acute phase, as well as to reduce the possibility of reverse causality.
Similar to previous studies,22 23 26 39 40 the present study demonstrated a greater risk of new DM also in the post-acute periods among hospitalized patients, with higher risk in those with greater COVID-19 severity. However, in contrast to previous studies,39 40 this study demonstrated no excess risk for new DM among 150 875 non-hospitalized patients with COVID-19. Moreover, by using several baseline times, this study demonstrated that the excess risk of DM among hospitalized patients was persistent but attenuated over time. The different findings among non-hospitalized patients may be related to differences in research methods and populations. Specifically, some previous studies assessed the association of COVID-19 with new DM in the context of long COVID-19.22 23 39 40 A study on US veterans included mostly white men with mean age of 61 years.26 A recent study on German individuals compared between patients with COVID-19 and individuals with acute upper respiratory tract infections.25 By using the integrated healthcare system’s electronic data warehouse of the largest healthcare organization in Israel, we were able to study a multiethnic large population, consisted of men and women with mean age of 43 years, and to follow them over the post-acute phase while focusing on incident DM as the main and sole outcome. Socioeconomic factors imposed by COVID-19 were considered in the analysis.
There are several limitations to this study. First is the observational nature of a retrospective study with a limited ability to ascribe a causal relationship. Second, increased interaction with the healthcare system for patients with COVID-19 may relate to increased opportunities to make a DM diagnosis. As expected, higher frequency of visits and blood glucose tests in the primary care clinics was observed among patients with COVID-19 compared with controls without COVID-19 during the follow-up period (98.2% and 57.5%, respectively). We partially addressed this potential surveillance bias by using 4-month landmark analysis, and diminished the difference in frequency of interaction with the healthcare system among patients with COVID-19 and non-COVID-19 matches (31.0% and 26.4%, respectively). Third, since the study was based on data retrospectively collected from the healthcare organization, the diagnosis of DM may have been missing or may not have been recorded accurately. To minimize the possibility of including undiagnosed individuals with DM, strict exclusion criteria were applied. Fourth, baseline variables were defined using the most recently available data prior to index date, and therefore, the data collected for some variables may not reflect the actual status at index date. This limitation is particularly relevant for variables such as smoking status, BMI, blood pressure, and laboratory data that can change over time. Fifth, classifying study population into COVID-19 and non-COVID-19 was based on documentation of a positive PCR test. There was a possibility of misclassifying undetected patients with COVID-19, especially those with asymptomatic and mild illness who did not perform the PCR test, into non-COVID-19 group. This limitation may result in a bias toward null. Sixth, data were collected before the Omicron variant emerged as a dominant variant of SARS-CoV-2 and therefore the results of this study may not apply to that variant. Seventh, data on the medication used, including corticosteroids, were available only in the community setting and were not available during hospitalization. Therefore, we were unable to adjust for or matched based on corticosteroid used during hospitalization. Lastly, in the frame of this study, it was not feasible to accurately break down the DM diagnosis data by type and distinguish between type 1 DM and T2DM.
Conclusions
Compared with individuals without COVID-19, non-hospitalized patients were not at higher risk of new DM either at the acute phase nor afterward. Hospitalized patients had a higher risk of new DM during the acute and post-acute phase, with the highest risk among severe hospitalized patients.
Data availability statement
No data are available. The data are not publicly available due to privacy restrictions.
Ethics statements
Patient consent for publication
Ethics approval
This study was approved by the Clalit institutional review board (approval number 0114-20-COM). Since the study was based on historical data from Clalit’s electronic data warehouse, individual patient consent was not required. All patient identities were concealed.
Acknowledgments
We thank Professor Morton Leibowitz for his thoughtful review. We thank Ms Noga Ramot, statistician and data analyst in the Branch of Planning and Strategy at Clalit Health Services, for her assistance in the statistical analyses.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
OR and TT contributed equally.
Contributors Study concept and design—OR, TT, AC, WS and GL. Acquisition analysis and interpretation of data—OR, TT, MH, AC, WS, PG, DD and GL. Drafting of the manuscript—OR and TT. Critical revision of the manuscript for important intellectual content—OR, TT, MH, AC, WS, PG, DD and GL. Statistical analysis—MH and OR. Administrative, technical and material support—OR, TT, MH and GL. Study supervision—GL. GL is the guarantor of the study.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.