Article Text
Abstract
Introduction The commensal bacterium Faecalibacterium prausnitzii is a prominent member of the microbiome of animals and humans, and it plays an important role in several physiological processes. Numerous studies have correlated the reduction of F. prausnitzii abundance with many disease states, including irritable bowel syndrome, Crohn’s disease, obesity, asthma, major depressive disorder, and metabolic diseases in humans. Studies have also correlated F. prausnitzii with diseases in humans involved in altered glucose metabolism, including diabetes.
Research design and methods The aim of this study was to investigate the effects of compositions derived from three strains of F. prausnitzii (coined FPZ) on glucose metabolism in diet-induced obese male C57BL/6J prediabetic and type 2 diabetic mice. The primary endpoints of these studies were measuring changes in fasting blood glucose, glucose tolerance (as measured by a glucose tolerance test), and percent hemoglobin A1c (HbA1c) with longer term treatment. Two placebo-controlled trials were carried out using both live cell FPZ and killed cell FPZ and extracts. Two additional placebo-controlled trials were carried out in non-diabetic mice and mice that previously had type 2 diabetes (T2D).
Results Both trials in prediabetic and diabetic mice revealed that peroral administration of live FPZ or extracts from FPZ lowered fasting blood glucose levels and improved glucose tolerance compared with control mice. A trial administering longer FPZ treatment also resulted in lowered percent HbA1c compared with control mice. Additionally, trials in non-diabetic mice treated with FPZ demonstrated that FPZ treatment does not lead to hypoglycemia.
Conclusions The trial results have shown that treatment with different formulations of FPZ result in lower blood glucose levels, lower percent HbA1c, and improved glucose response in mice compared with control prediabetic/diabetic mice. FPZ is a promising candidate as an orally administered probiotic or postbiotic to manage and improve pre-diabetes and T2D.
- Prediabetic State
- Glucose Tolerance Test
- Glycated Hemoglobin A
- Hypoglycemia
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
The abundance of commensal bacteria such as Faecalibacterium prausnitzii shows negative correlations with many disease states but lacks experimental data showing that administration of these live or killed beneficial bacteria will lead to health benefits, such as treatment of pre-diabetes and type 2 diabetes (T2D).
WHAT THIS STUDY ADDS
This study demonstrates that administering strains of the commensal F. prausnitzii or its extracts reduces fasting blood glucose, improves glucose response, and lowers percent HbA1c in prediabetic and diabetic mice without triggering hypoglycemia in mice with normal blood glucose levels.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study reveals the potential for products derived from F. prausnitzii or its extracts to be developed into treatments for pre-diabetes and T2D.
Introduction
Type 2 diabetes (T2D) and pre-diabetes are growing global health concerns. In 2019, an estimated 583 million people had diabetes worldwide (nearly 10% of the world’s adults), with T2D making up around 90%.1 T2D also rose from the 18th leading cause of death worldwide in 1990 to 9th in 2017.2 Pre-diabetes, an asymptomatic precursor to T2D, affects 84.1 million adult Americans (>30%), while another 28 million (>10%) have T2D.3 Pre-diabetes is characterized by insulin resistance, or a decrease in the body’s ability to use insulin signaling to uptake glucose, resulting in more insulin being needed to process the same amount of glucose as a healthy subject. This leads to elevated blood glucose levels.
In T2D, insulin production is no longer sufficient to compensate for insulin resistance and can be measured by a glucose tolerance test (GTT) in which glucose levels spike higher after a glucose challenge and take longer to return to pre-challenge levels than in insulin-sensitive individuals. Insulin resistance is also associated with small blood vessel disease,4 cardiovascular disease,5 non-alcoholic fatty liver disease,6 metabolic syndrome,7 and polycystic ovary syndrome.8 Adding to this health crisis, up to 70% of individuals with pre-diabetes will eventually develop diabetes in their lifetime.9 Alarmingly, efforts to slow or reverse the increasing prevalence of pre-diabetes and T2D have not been successful, with the rate of increase accelerating, especially in developed countries.10 As pre-diabetes and T2D continue to burden global public health systems, novel treatments to control and prevent pre-diabetes and T2D are urgently needed. Investigation of therapeutics with the ability to mitigate abrupt blood glucose spikes and lower elevated glucose levels is an important step to addressing this disease in both humans and animals.
Over the past several years, the gut microbiome has been linked to many human metabolic conditions including T2D.11 Elevated blood glucose has been associated with differences in the microbiome, with differences seen in the order, family, genus, species, and strain level between those in normal versus abnormal ranges. While causal relationships have not yet been established, early evidence shows that altering the microbiota by fecal transplantation can lead to improvements in insulin sensitivity and glucose metabolism.12 Faecalibacterium prausnitzii is a naturally occurring commensal bacterium found in the gastrointestinal tract of birds and mammals.13 14 In humans, F. prausnitzii comprises 3%–5% of the microbiome of a healthy gut and has been shown in numerous microbiome analyses to be lower in abundance in many disease states including irritable bowel disease, Crohn’s disease, asthma, depression, and metabolic disorders.15 16 Reduction of F. prausnitzii in the intestinal microbiota has been correlated with elevated blood glucose and T2D,17–28 and it has been found that different strains of F. prausnitzii may be present in healthy compared with disease states.29 30 From these observations, we hypothesize that administering specific strains of F. prausnitzii could modulate glucose metabolism and improve diabetic biomarkers. The aim of this study was to test whether live bacteria and/or bacterial extracts (postbiotics) from specific strains of F. prausnitzii31 have a positive impact on glucose metabolism in mice with diet-induced pre-diabetes or T2D. Both formulations have their benefits. Live cells have the potential to be incorporated into the microbiome and provide long-term excretion of effector molecules. However, as F. prausnitzii is an obligate anaerobe, the feasibility of its development into a probiotic is challenging, especially considering the costs associated with keeping anaerobes viable during processing and administration. If the molecules from cultures of F. prausnitzii are as effective as live F. prausnitzii, continuous administration of these products could represent a much more practical approach. We selected male C57BL/6J diet-induced obese (DIO) mice as a model system, as these mice have a genetic predisposition to develop elevated blood glucose, pre-diabetes, and ultimately non-insulin-dependent type II diabetes when fed a high-fat diet.32 The primary endpoints of these studies were changes in fasting blood glucose, glucose tolerance (as measured by GTTs), and percent HbA1c for longer term treatment.
Methods
FPZ production
Proprietary strains of F. prausnitzii were grown under anaerobic conditions using complex liquid media (coined FPZ media). DNA extraction was carried out using Instagene Matrix (Biorad, catalog no. 7326030), purified using a Wizard SV PCR clean up kit (Promega, catalog no. A9281), and the V1-V4 region of the 16S gene amplified by PCR (Promega, catalog no. M7822) and sequenced. Morphological observation and plating on selective media were also performed to confirm F. prausnitzii purity. To produce live cell formulations (FPZ-L), the fermentation product was directly lyophilized and stored under anaerobic conditions. Plate counts were conducted to determine colony-forming units (CFUs). Sequencing and growth on solid media were used to confirm pure cultures, as described above.
Mouse husbandry
Studies were conducted on C57BL/6J male mice purchased through Jackson Laboratories, under protocols approved by the UConn Health IACUC. All mice were allowed at least 1 week to acclimate to housing conditions before starting experiments.
All mice were housed in a temperature-controlled room with a 12-hour light-dark cycle and fed a high-fat diet (Research Diets) or regular chow and water ad libitum. All mice were cared for in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Mice were euthanized at the end of experiments by CO2 inhalation.
Powering
Powering was carried out for all trials using variance levels obtained from a previous study using C57BL/6J and C57BL/6J DIO mice.32 The study reported an 8% SD for C57BL/6J mice on a normal diet (same as mice used in study 3 and study 4), and 4% SD for C57BL/6J mice on a high-fat diet (same as mice used for study 1 and study 2). To be conservative and account for any potential extra variability, we set our SD at 10% of the mean. All trials were powered with at least the minimum number of mice per group to statistically detect a difference of 15% using an alpha of 0.05 and a powering of 80%.
Mouse trials
Mouse trials were carried out to investigate the effects of administering FPZ formulations in T2D mice (study 1), prediabetic mice (study 2), and normoglycemic mice (studies 3 and 4) to test the effects of FPZ in mice with different levels of glucose tolerance. Studies 1 and 4 investigated both live FPZ-L and postbiotic FPZ-S formulations, while studies 2 and 3 investigated only FPZ-S as this formulation is more amenable to repeated administrations as detailed in the Introduction section.
Four independent trials were conducted as outlined below:
Study 1: treatment of diabetic mice with live and killed cell FPZ formulations
Aims
To investigate whether administration of live and killed cell FPZ formulations results in differences to fasting blood glucose, percent HbA1c, and glucose response compared with placebo-treated mice using a T2D mouse model.
Primary outcomes
The primary outcomes for this study are the differences in fasting blood glucose, glucose response during a GTT, and percent HbA1c between mice treated with FPZ-L, or FPZ-S compared with placebo-treated control mice.
To determine if FPZ treatment can ameliorate T2D in obese mice, 27 male C57BL6/J mice were maintained on a high-fat diet (Research Diets, catalog no. D12492) for at least 3 months prior to the study. Mice were 38 weeks old at the time of enrollment. Mice were separated into three groups based on their weight, fasting glucose levels, and percent HbA1c prior to any intervention.
Treated mice as well as a control group were administered with either FPZ-L, FPZ-S, or deionized water as a control (n=9) via oral gavage for 14 consecutive days. Treatments were dissolved in deoxygenated deionized water at a concentration of 25 mg/mL and administered at a volume of 0.2 mL, with each mouse receiving 5 mg of product per day by gavage. For live cell treatments, this equates to 107 cells per treatment based on CFU counting described above. Control mice received an equal volume of deionized water. Prior to the start of treatment, baseline percent HbA1c values were taken using a Bayer DCA 2000+ blood analyzer to allow for longitudinal comparisons following the treatment period. Mice were monitored daily and weighed once per week. After 14 days of treatment, all mice were fasted for 16 hours followed by a GTT. Blood glucose measurements were recorded using an Accu-Chek Guide Me blood glucose meter. Glucose was administered at a concentration of 1 g/kg of mouse weight. Blood glucose measurements were taken at 0, 20, 50, 90, and 120 min timepoints. After all mice were treated for an additional 16 days (30 days total treatment), percent HbA1c values were collected.
Study 2: repeated FPZ-S treatment over three time periods with C57BL/6J DIO prediabetic mice
Aims
To investigate whether repeated administration of FPZ-S results in differences in fasting blood glucose or response to a GTT when compared with placebo-treated mice in a prediabetic mouse model.
Primary outcomes
The primary outcomes for this study are the differences in fasting blood glucose and glucose response between mice treated with FPZ-S compared with placebo-treated control mice.
To assess if FPZ-S treatment can improve elevated blood glucose levels associated with pre-diabetes and/or prevent the onset of T2D, 14 male 7-week-old C57BL/6J mice were enrolled in the study. Prior to the initiation of treatment, mice received a high-fat diet ad libitum to induce pre-diabetes. The high-fat diet was continued throughout the study. Mice were randomly allocated into one of two groups: placebo treatment (control) and killed-cell reconstituted lyophilized FPZ treatment (FPZ-S). Mice were acclimatized for 14 days by daily handling and administration of water by gavage. After acclimation, a daily dose of either treatment (FPZ-S, n=7) or control (control, n=7) was administered daily via oral gavage for 7-day, 10-day, or 15-day treatment periods throughout the 3-month trial. For the treatment group, FPZ-S was dissolved in saline at a concentration of 5 mg/mL and administered in a volume of 0.2 mL such that each mouse received 1 mg of product per day. Control mice received an equivalent volume of saline. On the final day of each treatment regimen, mice were fasted for 16 hours and a GTT was carried out as described in study 1.
Study 3: treatment of non-DIO mice with FPZ-S
Aims
To investigate whether administration of FPZ-S results in differences in fasting blood glucose and glucose response in normoglycemic mice.
Primary outcomes
The primary outcomes for this study are the differences in fasting blood glucose and glucose response between mice treated with FPZ-S compared with placebo-treated control mice.
To determine if FPZ-S treatment impacts normoglycemic animals, 14 male C57BL6/J mice were maintained on standard chow for 14 days. Mice were randomized into control (n=6) and FPZ-S treatment (n=8) groups. FPZ-S or placebo control was administered via oral gavage for 14 consecutive days. FPZ-S was dissolved in deionized water at a concentration of 25 mg/mL and administered at a volume of 0.2 mL, with each mouse receiving 5 mg of product per day. After 14 days of treatment, mice were fasted for 16 hours and GTTs were carried out as described in study 1.
Study 4: treatment of DIO mice after switching to a standard chow diet with FPZ
Aims
To investigate whether administration of FPZ-L or FPZ-S formulations results in differences in fasting blood glucose and percent HbA1c compared with placebo-treated mice in previously T2D mice.
Primary outcomes
The primary outcomes for this study are the differences in fasting blood glucose and percent HbA1c between mice treated with FPZ-L or FPZ-S compared with placebo-treated control mice.
To investigate the effect of FPZ during the transition between a high-fat and normal diet, 23 male C57BL/6J mice were maintained on a high-fat diet for 46 weeks and then switched to a standard diet for 30 days. Mice were separated into three groups consisting of a control group (n=8), an FPZ-L treated group (n=8), and an FPZ-S treated group (n=7). Each group was then treated for 28 days while maintaining consumption of the standard chow diet. Mice were then fasted for 16 hours, fasting blood glucose levels were recorded as described in study 1. Percent HbA1c levels were recorded immediately before diet change, immediately before commencement of treatment, and after the 1-month treatment period.
Statistical analysis
For studies with more than two groups (study 1 and study 4), one-way analysis of variance was carried out with an alpha of 0.05, followed by individual comparison tests between groups as outlined below. In studies with only two groups (study 2 and study 3), comparisons were carried out using unpaired t-tests. All comparisons were carried out with values of p<0.05 considered to be statistically significant.
Study 1
The comparisons carried out for study 1 are between FPZ-S or FPZ-L and control. The comparisons are at GTT timepoints 0, 20, 50, 90, and 120 min (shown in figure 1A), the total area under the curve (AUC) (figure 1B), and percent HbA1c after 30 days of treatment (figure 2A). The changes in fasting blood glucose and percent HbA1c over the course of treatment were also compared between FPZ-S or FPZ-L and control (online supplemental table 1 and figure 2B, respectively). The same comparisons were carried out between FPZ-S and FPZ-L, and no significant differences between these two groups were found in all cases.
Supplemental material
Effect of live or killed cell Faecalibacterium prausnitzii formulations (FPZ-L and FPZ-S) on glucose tolerance in diet-induced obese mice. C57BL/6J DIO male mice were treated with FPZ-S (n=9) or FPZ-L (n=9) or placebo (n=9) at 38 weeks of age for 14 days. After treatment, mice were fasted for 16 hours followed by a glucose tolerance test with (A) blood glucose levels measured over 2 hours and (B) total blood glucose area under curve (tAUC).
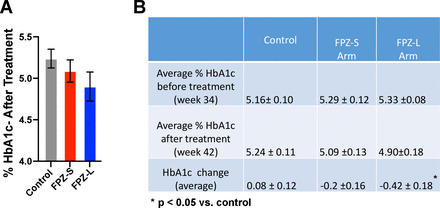
Effect of FPZ formulations on percent hemoglobin A1c levels in diet-induced obese mice. (A) Percent HbA1c after 30 days of treatment of 38-week-old male mice with FPZ-S (n=9) or FPZ-L (n=9) or placebo (n=9). (B) Comparison of percent HbA1c level before (week 34) and after (week 42) treatment with different formulations of FPZ.
Study 2
The comparisons carried out for study 2 are between FPZ-S and control. The comparisons are at 0, 20, 50, 90, and 120 min throughout the GTT assay, and incremental area under the blood glucose curve (iAUC) in three separate GTTs (shown in figure 3A–C, respectively) and total AUC (figure 3D) for each GTT.
Three phase FPZ-S treatment trials of C57BL/6J diet-induced obese mice. Male mice were treated with FPZ-S (n=7), or saline as a control (Control, n=7) followed by a 16-hour fast and glucose tolerance test. (A–C) Blood glucose levels and baselined incremental AUCs (iAUCs) are shown. The age of mice at the start of treatment and treatment time were as follows: (A) Phase A—11-week-old mice, 7 days of treatment; (B) Phase B—18-week-old mice, 14 days of treatment; (C) Phase C—23-week-old mice, 10 days of treatment. As shown in (D), tAUC decreased after three FPZ-S treatments, while tAUC increased in control mice, suggesting repeated FPZ-S treatment is having a long-term effect on glucose metabolism in mice. AUC, area under the curve.
Study 3
The comparisons carried out for study 3 are between FPZ-S and control. The comparisons are at GTT timepoints 0, 20, 50, 90, and 120 min and total AUC from this GTT (figure 4).
FPZ-S does not lead to hypoglycemia in non-diabetic mice. Eight non-diabetic male mice treated for 14 days with FPZ-S showed comparable fasting blood glucose and response during a glucose tolerance test versus control mice with both glucose spike and area under the curve not differing statistically. The dashed line represents the lower limit of normal glycemic levels in mice (80 mg/dL). Blood glucose levels below this level are indicative of hypoglycemia.
Study 4
The comparisons carried out for this study are between FPZ-S or FPZ-L and the control. The comparisons are fasting blood glucose and percent HbA1c after 28 days of treatment (figure 5A,B, respectively). The same comparisons were carried out between FPZ-S and FPZ-L and no significant differences were found in both cases.
Treatment with FPZ formulations does not lead to hypoglycemia in previously diabetic mice. In 23 male mice that had been switched from high-fat to standard chow diets, levels of (A) fasting blood glucose and (B) percent HbA1c were not significantly lower in mice treated for 28 days with two formulations of FPZ versus the control, indicating that while FPZ treatment leads to lower glucose levels in diet-induced obese mice, it does not lead to hypoglycemia in non-diabetic mice. The dashed line represents the lower limit of normal glycemic levels in mice (80 mg/dL). Blood glucose levels below this level are indicative of hypoglycemia.
Results
Study 1: effect of live and killed cell FPZ on mice with T2D
Both FPZ-S and FPZ-L treated groups displayed statistically significant lower fasting blood glucose levels following 14 days of treatment when compared with control mice (p<0.01), as shown in figure 1A. Following glucose administration, treatment of T2D mice with either of the tested formulations resulted in a statistically significant lower total blood glucose (tAUC) in the 2 hours after a GTT compared with control (p<0.05 FPZ-S, p<0.01 FPZ-L), as shown in figure 1B. Also, as shown in online supplemental table 1, while control mice showed increases in blood glucose levels during the treatment period (an average increase of 28 mg/dL), all treated mice showed a reduction in blood glucose levels during this period (an average of 9 and 14 mg/dL for FPZ-S and FPZ-L, respectively; both p<0.01 compared with control). Mice in both treatment groups had a lower percent HbA1c than controls 30 days after the start of treatment (figure 2A), although this trend was not statistically significant. Both groups of FPZ-treated mice experienced a decrease in percent HbA1c during treatment, while the control group showed an increase (figure 2B), with the difference between FPZ-L and control being statistically significant (p<0.05). No statistically significant differences were observed between FPZ-L and FPZ-S treatments when any of the above comparisons were made between treatment groups.
Study 2: effects of repeated treatment of prediabetic mice with FPZ-S
In the first phase of this study, 11-week-old prediabetic C57BL/6J DIO mice (fasting blood glucose between 100 and 200 mg/dL) treated with FPZ-S for 7 days showed significantly lower blood glucose spike in the 2 hours following glucose challenge compared with control (as shown in figure 3A, iAUC, p<0.05) indicating that FPZ-S improved glucose tolerance. In the second phase, the effect of FPZ-S treatment on 18-week-old mice that had developed T2D (fasting glucose >200 mg/dL) was tested with the results shown in figure 3B. Similar to the results seen in the first phase, FPZ-S treatment of T2D mice resulted in a significantly lower blood glucose spike in the 2 hours after a GTT compared with control (iAUC, p<0.05). The blood glucose levels of FPZ-S-treated mice also returned to baseline fasting levels, while blood glucose levels in control mice remained elevated after 2 hours. Treatment phase 3 was carried out on 23-week-old mice with more advanced T2D (as represented by more highly elevated blood glucose levels), as shown in figure 3C. Mice were treated with FPZ-S for 10 days followed by a GTT. For these DIO mice with more advanced T2D, a significantly lower glucose spike was seen compared with control (iAUC, p<0.01). Additionally, significantly lower blood glucose levels were seen with fasting and at every time point for 2 hours after GTT compared with control, with blood glucose levels returning to baseline faster in FPZ-S treated versus control mice. Total blood glucose, as measured using tAUCs, for FPZ-S treated mice was found to be 34% lower than control (p<0.01), as shown in figure 3D.
Study 3: effect of FPZ-S on non-diabetic mice
To test the effect of FPZ-S on healthy mice with normal blood glucose levels, a trial was performed using C57BL/6J normoglycemic mice reared with standard chow. After 14 days of treatment, a GTT was performed, and blood glucose levels were measured. As shown in figure 4, mice treated with FPZ-S had comparable fasting blood glucose levels compared with control mice and did not differ in GTT response when compared with control mice (less than a 15% difference based on the level of powering). The fact that these mice did not show lower blood glucose levels indicates that FPZ-S does not perturb glucose metabolism in healthy mice, unlike some other antidiabetic therapies.33
Study 4: effect of FPZ-S and FPZ-L on previously diabetic mice
As diabetic treatment is most effective when coupled with improvements in lifestyle and diet, we tested the effects of FPZ-L and FPZ-S on blood glucose levels of DIO mice that had been switched to a standard diet to ensure FPZ-L or FPZ-S did not lead to hypoglycemia in previously diabetic mice with normal fasting blood glucose levels. As shown in figure 5, after 28 days of treatment, mice in both treatment groups had comparable fasting blood glucose levels and percent HbA1c to control mice. This indicates that neither FPZ formulation led to hypoglycemia in previously diabetic mice.
Discussion
The current study investigates two potential products to ameliorate elevated blood glucose levels in pre-diabetes and T2D: a probiotic live cell product (FPZ-L) and postbiotic extract (FPZ-S). As pre-diabetes and T2D are chronic conditions, the aims of this study were to evaluate the effects of FPZ on prediabetic and T2D mice, looking specifically at changes in fasting glucose, glucose response, and changes in percent HbA1c in mice treated for longer periods (30 days treatment, study 1).
In study 1, the treatment of mice with T2D with either FPZ-L or FPZ-S resulted in significantly lower fasting glucose and improved glucose response, as shown in figure 1. In the same study, lower percent HbA1c was also seen with numerically lower percent HbA1c after treatment with either FPZ-S or FPZ-L, as shown in figure 2A, while percent HbA1c was seen to decrease with both treatments compared with control (significant for FPZ-L (p<0.05), non-significant for FPZ-S). As percent HbA1c reflects the average blood glucose level over long time periods (half-life of red blood cells in mice is 45 days),34 the trend of lower percent HbA1c after only 28 days is encouraging. As F. prausnitzii is an obligate anaerobe, the feasibility of its development into a probiotic is challenging considering the costs associated with keeping anaerobes viable during processing and administration.
If the molecules from cultures of F. prausnitzii are indeed as effective as live F. prausnitzii, continuous administration of these products could represent a much more practical therapeutic approach. We therefore tested the effect of repeated administration of FPZ-S on prediabetic mice in study 2. As well as seeing lower blood glucose levels and improved glucose response similar to study 1, the data from study 2 suggest that repeated administration of FPZ-S resulted in long-term benefits. This is evidenced by the fact that fasting glucose levels and glucose spikes during GTTs in control mice increased over time (progressing from pre-diabetes to T2D), while fasting glucose levels of FPZ-S-treated mice and glucose spikes were lower in the 23-week-old mice. This is supported by our analysis of tAUCs after GTT for the mice of different treatment phases and suggests that repeated FPZ-S treatments have a sustained positive effect on glucose tolerance in mice with repeated administration (figure 3D) and have the potential to be used as an alternative to live cell probiotics.
It is also important that diabetic treatments do not lower blood glucose below normal levels leading to hypoglycemia. In studies 3 and 4, we demonstrated that FPZ treatment did not lower fasting blood glucose levels below the normal range in mice (a lower limit of 80 mg/dL), demonstrating that FPZ treatment does not lead to hypoglycemia.
For this study, we chose male C57BL/6 DIO mice, as they are the most representative mouse model for studying pre-diabetes and T2D. One limitation to using the DIO model is that only male mice can be used, as only males develop significant weight-associated comorbidities, such as diabetic disease.35 To assess the broader potential of FPZ to treat pre-diabetes, T2D, gestational diabetes, and even type 1 diabetes, further trials will be needed using different model systems with additional parameters measured such as insulin levels and other diabetic biomarkers. Although the current study did not investigate the effect of diet composition (the DIO model was used to generate the phenotype of elevated blood glucose and insulin resistance), we plan to carry out future studies to assess the effect of FPZ in animals on a high-sugar diet.
Pre-diabetes and T2D are critical public health concerns whose burdens are increasing in many countries around the world. The need for novel low-cost therapeutics to prevent and treat these conditions is urgent, as current interventions are not slowing the increase in pre-diabetes and T2D prevalence. Additionally, current treatments, while in some cases effective, have many drawbacks including off-target effects, risk of hypoglycemia, adverse drug reactions, and other treatment-limiting side effects. The current first-line drug for pre-diabetes and T2D in humans is metformin, the fourth most prescribed drug in America.36 However, approximately 20% of metformin patients experience gastrointestinal side effects, resulting in poor patient adherence.37 More importantly, many patients taking metformin monotherapy cannot reach or maintain healthy HbA1c levels, with an annual failure rate of up to 17% per year in clinical practice settings.38 These patients often require two or more antidiabetic drugs to control their blood glucose levels. While future studies are needed to elucidate the mechanism by which FPZ leads to increased glucose tolerance and lowering of blood glucose levels, there is a need for novel therapeutics to treat the growing incidences of pre-diabetes and T2D.
The current study shows that daily FPZ administration leads to significantly lower fasting blood glucose, decreased percent HbA1c levels, and significantly improved glucose response. Moreover, the long duration trial with multiple rounds of FPZ treatment (study 2) showed long-lasting glycemic control. Additionally, the trials carried out on non-diabetic mice and mice that were previously diabetic demonstrate that treatment with FPZ only leads to the lowering of elevated blood glucose levels and does not trigger hypoglycemia. While additional preclinical and clinical studies are needed, we are optimistic that using FPZ as either a probiotic or postbiotic could provide a safe alternative to prevent or ameliorate preexisting pre-diabetes and T2D.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
The authors wish to thank Dr Ryan Clauson for his helpful comments on this manuscript.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors Jk and SAM are guarantors of this work. JK and SAM conceived and designed the experiments. JK, EG, and SAM carried out the experiments. JK and SAM wrote the manuscript. JK, EG, LKS, and SAM edited the original manuscript. JK, DR, and SAM revised the manuscript. JK, DR, LKS and SAM edited the revised manuscript. All authors read and approved the manuscript.
Funding This research was funded by Bactana Corp., Farmington, CT.
Competing interests Bactana Corp. has received regulatory approvals for FPZ-S and FPZ-L (ID 8626) through Health Canada’s Veterinary Health Program as active ingredients for pets and livestock. JK and SAM are the inventors of the patent WO2022026873A1 (filed July 30, 2021, published February 3, 2022). JK, DR, and SAM are employees and stockholders of Bactana Corp.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.