Article Text
Abstract
Introduction Previous studies have suggested that maternal diabetes may have programming effect on fetal brain development. However, little is known about the association between maternal diabetes and neurodevelopmental disorders in offspring that mainly manifest in infancy or early childhood. We aimed to examine the association between maternal diabetes before or during pregnancy and feeding and eating disorders (FED) in offspring.
Research design and methods This population-based cohort study included 1 193 891 singletons born in Denmark during 1996–2015. These children were followed from birth until the onset of FED, the sixth birthday, death, emigration, or 31 December 2016, whichever came first. Relative risk of FED was estimated by HRs using Cox proportional hazards model.
Results A total of 40 867 (3.4%) children were born to mothers with diabetes (20 887 with pregestational diabetes and 19 980 with gestational diabetes). The incidence rates of FED were 6.8, 4.6 and 2.9 per 10 000 person-years among children of mothers with pregestational diabetes, gestational diabetes and no diabetes, respectively. Offspring of mothers with diabetes had a 64% increased risk of FED (HR 1.64; 95% CI 1.36 to 1.99; p<0.001). The HR for maternal pregestational diabetes and gestational diabetes was 2.01 (95% CI 1.59 to 2.56; p<0.001) and 1.28 (95% CI 0.95 to 1.72; p=0.097), respectively. The increased risk was more pronounced among offspring of mothers with diabetic complications (HR 2.97; 95% CI 1.54 to 5.72; p=0.001).
Conclusions Maternal diabetes was associated with an increased risk of FED in offspring in infancy and early childhood. Our findings can inform clinical decisions for better management of maternal diabetes, in particular before pregnancy, which can reduce early neurodevelopmental problems in the offspring.
- diabetes
- gestational
- diabetes complications
- mental disorders
- feeding and eating disorders
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Significance of this study
What is already known about this subject?
The diabetic intrauterine environment can interfere with fetal brain development, which would contribute to an increased susceptibility to neurodevelopmental disorders.
However, little is known about the association between maternal diabetes during pregnancy and the risk of neurodevelopmental disorders that mainly manifest in infancy or early childhood.
What are the new findings?
Maternal diabetes before or during pregnancy was associated with an increased risk of feeding and eating disorders in offspring.
The highest risk of feeding and eating disorders was observed among offspring of mothers with diabetic complications.
How might these results change the focus of research or clinical practice?
Early screening and treating diabetes in women during their childbearing age could be important to reduce neurodevelopmental disorders in offspring.
Introduction
Feeding and eating disorders (FED) are one of the most common neurodevelopmental disorders during infancy and early childhood,1 affecting approximately 0.8%–1.4% of the normally developing children.2 3 FED are characterized by persistent eating disturbances, such as refusal of food and extreme faddiness, and eating-related problems, such as eating of non-edible substances, or voluntary regurgitation of foods after eating in the absence of nausea, involuntary retching, or disgust.4 Children with FED often demonstrate lower performances in neuropsychological assessments of executive functioning, visuospatial memory, as well as central coherence.5 The etiology of FED is poorly understood, resulting in difficulties in disease prevention and management.6
It has been proposed that prenatal risk factors may be involved in the development of FED.1 7 Emerging evidence suggests that adverse intrauterine environment may play a role in the development of neurodevelopmental disorders.8 9 Maternal diabetes during pregnancy may exert long-lasting effects on organ development and function in offspring.10 11 The diabetic intrauterine environment can interfere with fetal brain development,12 13 which would contribute to an increased susceptibility to neurodevelopmental disorders.14 15 Experimental studies have suggested that hyperglycemia during early embryogenesis can disturb neocortical neurogenesis via epigenetic regulations.16 17 For example, in a mouse model of a hyperglycemia, it was observed that maternal hyperglycemia could alter histone acetylation and its regulation on the transcription of proneural genes that were associated with disrupted differentiation of neural stem cells and newborn projection neurons in the neocortex.16 Epidemiological studies have shown that maternal pregestational type 1 and type 2 diabetes and gestational diabetes mellitus (GDM) are associated with an increased risk of several neurodevelopment disorders in childhood or adolescence, such as autism spectrum disorders (ASD) and attention-deficit/hyperactivity disorders.15 18–20 However, little is known about the association between maternal diabetes and the risk of FED that mainly manifests in infancy or early childhood.
We hypothesized that intrauterine exposure to maternal diabetes contributes to an increased risk of FED in offspring. As the odds of neurodevelopment disorders varies by types of maternal diabetes,18 19 we conducted a large cohort study to examine the associations between specific types of maternal diabetes and FED in offspring and whether diabetic complications during pregnancy further increased the risk of FED.21 22
Research design and methods
Data source and cohort identification
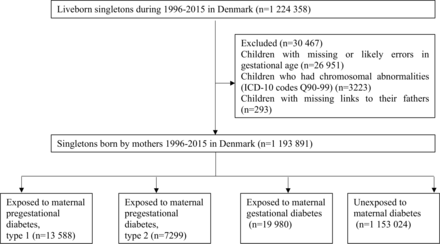
We conducted this secondary analysis of previously collected data of the Danish national registers, such as the Medical Birth Registry, the National Patient Register, the National Prescription Register, and the Psychiatric Central Research Register.23–27 In Denmark, all live births have a unique personal identification number which permits the accurate linkage of individual-level data. We identified all singleton live births from 1 January 1996 to 31 December 2016 (n=1 224 358) from the Danish Medical Birth Registry,23 excluded 26 951 children who had missing or extreme gestational age (<154 or >315 days), 3223 children with chromosomal abnormalities, and 293 children without links to their fathers. The final analysis included 1 193 891 children (shown in figure 1). We followed each child from birth until the date of the first diagnosis of FED, date of sixth birthday, emigration, death, or end of follow-up (31 December 2016), whichever came first.
Flow chart showing the identification of the eligible participants and analysis sample. ICD-10, International Classification of Diseases 10th Revision.
Measures
Maternal diabetes
Information on maternal diabetes was obtained from the Danish National Patient Register (DNPR) including all inpatient and outpatient records,24 the Danish National Diabetes Register,28 and the National Prescription Register,27 using International Classification of Diseases codes (ICD-8 codes during 1970–1993 and ICD-10 codes since 1994) and Anatomical Therapeutic Chemical (ATC) classification codes. In Denmark, the diagnostic criteria for diabetes are as follows: fasting plasma glucose ≥7.0 mmol/L or 2-hour postload plasma glucose ≥11.1 mmol/L or HBA1c ≥6.5%.29–31 According to local screening procedures, GDM was diagnosed32 when two or more glucose values exceeded the following capillary whole blood values on oral glucose tolerance test results: 5.7 mmol/L at 0 min, 11.9 mmol/L at 30 min, 12.0 mmol/L at 60 min, 9.7 mmol/L at 90 min, 8.9 mmol/L at 120 min, 8.5 mmol/L at 150 min and 7.4 mmol/L at 180 min. Maternal diabetes before childbirth was categorized as pregestational diabetes and gestational diabetes. Pregestational diabetes was ascertained using the following criteria: (1) diagnosis of diabetes (ICD-8 codes: 249, 250; ICD-10 codes: E10–E11, H36.0, O24 excluding O24.4 and O24.9); (2) receipt of chiropody for patients with diabetes; (3) two redeemed prescriptions for insulin (ATC code: A10A); or (4) two redeemed prescriptions for oral antidiabetics (ATC code: A10B), and type 2 diabetes (ICD-8: 250).22 33 Pregestational diabetes was further classified as type 1 diabetes (ICD-8 code: 249; ICD-10 codes: E10, O24.0; ATC codes: A10A) or type 2 diabetes (ICD-8 code: 250; ICD-10 codes: E11, O24.1; ATC code: A10B).22 33 During 1977–1986 in Denmark, type 1 and type 2 diabetes were recorded using the single ICD-8 code (250).24 We used two approaches to distinguish between type 1 and type 2 diabetes: (1) a specific code for type 1 or type 2 diabetes registered later; or (2) age of diabetes onset (type 1: <30 years and type 2: ≥30 years), as in other studies.21 22 33
Gestational diabetes has been coded independently throughout the study period (ICD-8: 63474, Y6449; ICD-10: O244, O249). If a mother was recorded with different diabetes types at various hospital contacts, she was classified based on the first diagnosis type. Diabetic complications, such as diabetic coma, ketoacidosis, nephropathy, ophthalmic, neurological, circulatory, and unspecified, or multiple complications, could in part reflect poor glycemic regulation.21 22 We used DNPR to identify mothers with pregestational diabetes who had diabetic complications (ICD-10 codes: E100–E108, E110–E118, and H36.0).
Feeding and eating disorders
FED, specific to infancy and early childhood, are characterized by refusal of food and extreme faddiness in the presence of an adequate food supply, a reasonably competent caregiver, with the absence of organic disease, and behavioral problems such as eating of non-edible substances (pica) or voluntary regurgitation of foods (rumination-regurgitation disorder).3 FED should be diagnosed only if the difficulties are clearly beyond the normal range, if the nature of the eating problem is qualitatively abnormal in character, or if the child fails to gain weight or loses weight over a period of at least 1 month.3 Information on FED diagnosed before 6 years old was obtained from the DNPR and the Danish Psychiatric Central Research Register,24 25 which hold all inpatient and outpatient psychiatric disorders diagnosis in Denmark. The ICD-10 codes for FED were F98.2 and F50.8.1
Covariates
Based on previous research and according to directed acyclic graphs (online supplemental figure 2),1 15 19 the following factors were considered as potential confounders and included in the adjusted models: gender of the child (male, female), calendar period of birth (a 5-year interval during 1996–2015), parity (1, 2, ≥3), maternal age at birth (≤25, 26–30, 31–35, ≥36 years), paternal age at birth (≤25, 26–30, 31–35, ≥36 years), maternal country of origin (Denmark, other countries), maternal education level (0–9, 10–14, ≥15 years), maternal cohabitation status (yes, no), maternal smoking status (yes, no), maternal psychiatric disorder history (yes, no), and paternal psychiatric disorder history (yes, no). The information for maternal social status and origin of country was obtained from the Danish Integrated Database for Longitudinal Labor Market Research.34
Supplemental material
Statistical analysis
We used Cox proportional hazards regression model to estimate the HR with 95% CIs for the association of maternal diabetes with the risk of FED in offspring, with offspring’s age as the timescale. We categorized mothers with diabetes into four groups: no diabetes, type 1 diabetes, type 2 diabetes and GDM. The mothers with no diabetes were included in the reference group. To control for the correlations of sequential birth of the same mother, the robust sandwich estimator for SE was used. Furthermore, we examined the association between maternal pregestational diabetes and FED in offspring stratified by maternal diabetes complications.
We performed two models for adjusting potential confounders. Model 1 was adjusted for birth of year. Model 2 was additionally adjusted for sex, parity, parental age at birth, maternal education level, maternal origin, maternal cohabitation, maternal smoking during pregnancy, and parental psychiatric disorders before the childbirth.
We performed the causal inference methods for mediation analyses to examine whether adverse birth outcomes (low birth weight, preterm birth, low Apgar score, and small for gestational age) mediated the association between maternal diabetes and FED in offspring by calculating direct and indirect effects (via the mediator) in the STATA module PARAMED.35 The proportion of mediation was calculated as log (natural indirect relationship)/log (total relationship).
We did several sensitivity analyses. First, in order to examine the potential mediating effects of preterm birth, we repeated the analyses only including term-born children (≥37 gestational weeks) to see whether the associations would be changed significantly, compared with those overall estimates. Second, we also examined the paternal diabetes as the exposure to explore the potential roles of genetic components and family environmental factors. Paternal diabetes was identified with the same strategy as for maternal diabetes. Additionally, we also conducted cosibling analyses to explore potential confounding by shared familial factors (genetic and/or environmental).36 Third, as maternal pregnancy body mass index became available since 2004, we restricted the analysis to offspring born afterward. Fourth, we repeated our analysis by excluding children born with cleft lip and cleft palate (Q35–Q37), and other congenital malformations of the digestive system (Q38–Q45). Fifth, we also conducted analyses by extracting information on maternal diabetes through patient registers only rather than combination of hospitalization registers and prescription data. Finally, we examined potential sex-specific associations between maternal diabetes and FED in offspring by including an interaction term between sex of the child and maternal diabetes. All statistical analyses were performed with Stata V.15.
Results
Of the 1 193 891 singleton live-born offspring, 20 887 (1.7%) were exposed to maternal pregestational diabetes (type 1: 1.1%, type 2: 0.6%) and 19 980 (1.7%) were exposed to gestational diabetes. The proportion of offspring exposed to maternal diabetes increased with the year of birth (online supplemental figure 1). Table 1 shows the characteristics of mothers and children in the exposed and unexposed groups. Compared with unexposed offspring, exposed offspring were more likely to be born preterm. Compared with mothers without diabetes, mothers with diabetes were on average older, more likely to have higher parity, to live alone, and be non-smokers during pregnancy. Mothers with diabetes also had a higher prevalence of psychiatric disorders.
Characteristics of the study population born between 1996 and 2015 at birth according to maternal diabetes status
A total of 2099 (0.2%) offspring were diagnosed with FED with a mean age at diagnosis of 1.2 years. Offspring of women with diabetes were 1.64 times (HR 1.64; 95% CI 1.36 to 1.99; p<0.001) more likely to have FED than the offspring of mothers without diabetes (incidence rate of FED 0.57 per 1000 person-years vs 0.29 per 1000 person-years). For example, the rate of 0.57 per 1000 person-years could be considered that when we follow 100 000 children for 1 year, 57 of them will develop FED. The magnitude of association was higher in offspring exposed to maternal pregestational diabetes (HR 2.01; 95% CI 1.59 to 2.56; p<0.001) than in those exposed to gestational diabetes (HR 1.28; 95% CI 0.95 to 1.72; p=0.097). Both maternal pregestational type 1 diabetes (HR 1.73; 95% CI 1.26 to 2.38; p=0.001) and type 2 diabetes (HR 2.53; 95% CI 1.78 to 3.59; p<0.001) were associated with an increased risk of FED in offspring (table 2).
Association between maternal diabetes and risk of feeding and eating disorders in offspring
Among women with pregestational diabetes, 16.6% had pregestational diabetic complications. The risk of FED in offspring of mothers with pregestational diabetes and diabetic complications was 2.97-fold (HR 2.97; 95% CI 1.54 to 5.72; p=0.001) than those of mothers with pregestational diabetes but without diabetic complications (HR 1.92; 95% CI 1.49 to 2.48; p<0.001) (table 3).
Association between maternal diabetic complications and risk of feeding and eating disorders during 1996–2015
Adverse birth outcomes accounted for a very small proportion of the association between maternal diabetes and risk of FED, although almost all the natural indirect association estimates were marginally statistically significant (table 4).
Mediation analysis with adverse birth outcomes as potential mediators between maternal diabetes and risk of feeding and eating disorders in offspring
When excluding offspring with preterm birth, the estimates remained unchanged (online supplemental table 1). No association was observed for paternal diabetes and FED risk in offspring (online supplemental table 2). In the cosibling analyses, similar association was yielded (online supplemental table 3). Results from the analyses restricted to offspring born after 2004 or excluding children born with cleft lip and cleft palate, and other congenital malformations of the digestive system were consistent (online supplemental tables 4 and 5). Stratification for sex of the offspring did not show any significant differences in estimates from the main analyses (online supplemental table 6). When obtaining information on maternal diabetes only from patient registers, results were similar (online supplemental table 7).
Discussion
In this large cohort study, we found that maternal diabetes before or during pregnancy was associated with an increased risk of FED in offspring. Children of mothers with pregestational diabetes (type 1 or type 2 diabetes) had varied increased risks (1.73 for type 1; 2.53 for type 2) than those of mothers without diabetes, which were higher than that in children of mothers with GDM only. The highest risk of FED was observed among offspring of mothers with diabetic complications. Mediation analyses further indicated that adverse birth outcomes could only explain a very small proportion of the overall effects.
Our findings are, in general, consistent with previous studies on the association between maternal pregestational diabetes and neurodevelopmental disorders in childhood or adolescence,14 15 18–20 37 38 but it is new to observe the elevated risk in infancy and early childhood. We further observed that the associations of type 1 and type 2 diabetes with FED might vary in magnitude. The variations in the magnitude of the FED risk in offspring by subtypes of maternal diabetes could be due to the different pathophysiological mechanisms underlying subtypes of diabetes,39 which warrants further investigations.
Regarding GDM, a meta-analysis of both prospective and cross-sectional studies reported that GDM was associated with ASD in offspring.40 However, a large population-based study in the USA found that only GDM diagnosed before 26 weeks was significantly associated with risk for ASD.19 Our stratified analyses found that children exposed to GDM diagnosed before 26 weeks had a similar HR, compared with children exposed to GDM at any time. Several register-based studies have suggested that the association between GDM and neurodevelopmental disorders in offspring was only observed in children of parents with lower socioeconomic position or of mothers with obesity.14 18 41–43 These discrepancies might be explained by different screening procedures, diagnostic criteria, and treatment guidelines for GDM, or measurements for offspring neurodevelopment.40
Potential biological mechanisms linking maternal diabetes during pregnancy and FED risk in offspring may involve multiple pathways. During diabetic pregnancies, maternal hyperglycemia may predispose fetuses to a proinflammatory state with fetal hyperinsulinemia,44 increased oxidative stress,45 chronic inflammation,11 and hypoxia,46 which in turn could interfere with brain development during the critical period and lead to subsequently neurobehavioral disorders in later life.12 47 Previous studies have established that the development of neurons and brain circuits, that is, proliferation, migration, differentiation, is an array of complex processes, and are more susceptible to environmental insults during early pregnancy.48 49 During this period, maternal hyperglycemia may play a more critical role in the etiology of neurodevelopment disorders.50 51 Maternal hyperglycemia has also been associated with epigenetic modifications which potentially mediate the link between maternal diabetes and FED risk in offspring.52 Experimental studies in rats have suggested that epigenetic modifications of neocortical neurogenesis due to alterations in the hyperglycemic intrauterine environment increased the susceptibility to neurodevelopment disorders in later life.16 In addition, infants born to mother with diabetes have higher serum leptin and Mendelian randomization supports the causal relationship between maternal hyperglycemia and epigenetic regulation of leptin gene in newborns.53 Leptin, one of the anorexigenic neurotransmitters, may restrain the feeding behavior by restricting the availability or counteracting the orexigenic effect of neuropeptide Y,54 which may partly explain the disturbed feeding behaviors in offspring of mothers with diabetes.
It is noteworthy that children of mothers with diabetic complications have the highest risk of FED. Diabetic complications are closely correlated with insulin resistance and may reflect the severity of pregestational diabetes and poor glycemic control.55 Similarly, previous studies have found a higher risk of genital anomalies and cardiovascular diseases in offspring of mothers with diabetic complications.21 33 Maternal poor glycemic control in pregnancy may exert potential implications in offspring for behavioral and emotional problems.56 57
Strengths and limitations of this study
This study has several strengths. First, methodological strengths of this study include its use of the Danish register data, which provide a large and representative sample with more than 1 million children. Second, in this study, we have used Cox proportional hazards model. This statistical model can take into consideration of time until FED occur and compare the incidence rate of events over time for different groups, while adjusting for various potential confounders. Third, the data used in this study were extracted from national registers which have been proved to be reliable and of high quality.24 25 28 Furthermore, the data are prospectively collected, so the possibility of recall bias could be ruled out.
Our study also had several limitations. First, the FED treated in private clinics could not be included. However, misclassification of FED might be expected to be non-differential by maternal diabetes and therefore are more likely to influence our results toward the null. Second, the potential misclassification bias might also remain for maternal diabetes. Before 1986, type 1 and type 2 diabetes were recorded using the same ICD-8 codes.28 We further validated the diagnosis by using the specific register information for diabetes later and previous studies have indicated the high validity of Danish diabetic diagnosis in epidemiological studies. Third, although we adjusted for relevant confounders, as in other observational studies, we cannot completely rule out the possibility of residual confounding by unmeasured confounders. However, the cosibling analyses showed that they were not explained by unmeasured shared familial factors. In addition, no significant association between paternal diabetes further suggested the findings are unlikely to be explained by the uncontrolled confounding completely. Lastly, pregestational diabetes with complications could indicate severity of diabetes, poor glycemic control or duration of exposure to hyperglycemia. However, due to lack of detailed information on these factors, we could not explore further on this issue and further research is warranted.
Conclusion
Maternal diabetes was associated with an increased risk of FED in offspring in infancy and early childhood. Our findings highlight the importance of better management of maternal diabetes, which may contribute to reduce the incidence of neurodevelopmental problems in the offspring like FED.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors HW preformed the literature review, conducted data analyses and drafted the manuscript. HH, YY, and XS contributed to the interpretation of the data, critical revision of the paper and approval of the final version. HW, FL and JL are the guarantors of the study. They developed the study conception, directed the analytic strategy of the study and supervised the drafting of the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Funding This study was funded by the National Nature Science Foundation of China (No 81703237, No 81930095, No 81761128035, No 81701334, No 81703249), the Shanghai Municipal Commission of Health and Family Planning (No 2017ZZ02026, No 2018BR33, No 2017EKHWYX-02), the Shanghai Shenkang Hospital Development Center (No 16CR2025B), the Shanghai Clinical Key Subject Construction Project (shslczdzk02902), the Shanghai Committee of Science and Technology (No 17XD1403200, No 19410713500, No 18DZ2313505), the Shanghai Municipal Science and Technology Major Project (No 2018SHZDZX01), the Key Scientific and Technological Projects of Guangdong Province (2018B030335001), the Collaborative Innovation Program of Shanghai Municipal Health Commission (2020CXJQ01), the National Human Genetic Resources Sharing Service Platform (2005DKA21300), the Xinhua Hospital of Shanghai Jiao Tong University School of Medicine (2018YJRC03), the Novo Nordisk Foundation (NNF18OC0052029), the Danish Council for Independent Research (DFF-6110-00019B and 9039-00010B), the Nordic Cancer Union (176673, 186200, and R217-A13234-18-S65), and the Karen Elise Jensens Fond (2016).
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval The study was approved by the Danish Data Protection Agency (No 2013-41-2569) and the Danish Health Data Authority.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement No data are available. Data are not publicly available.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.